Covid news – live: EU withdraws controversial plan for vaccine export controls at Irish border
Follow for the latest developments
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
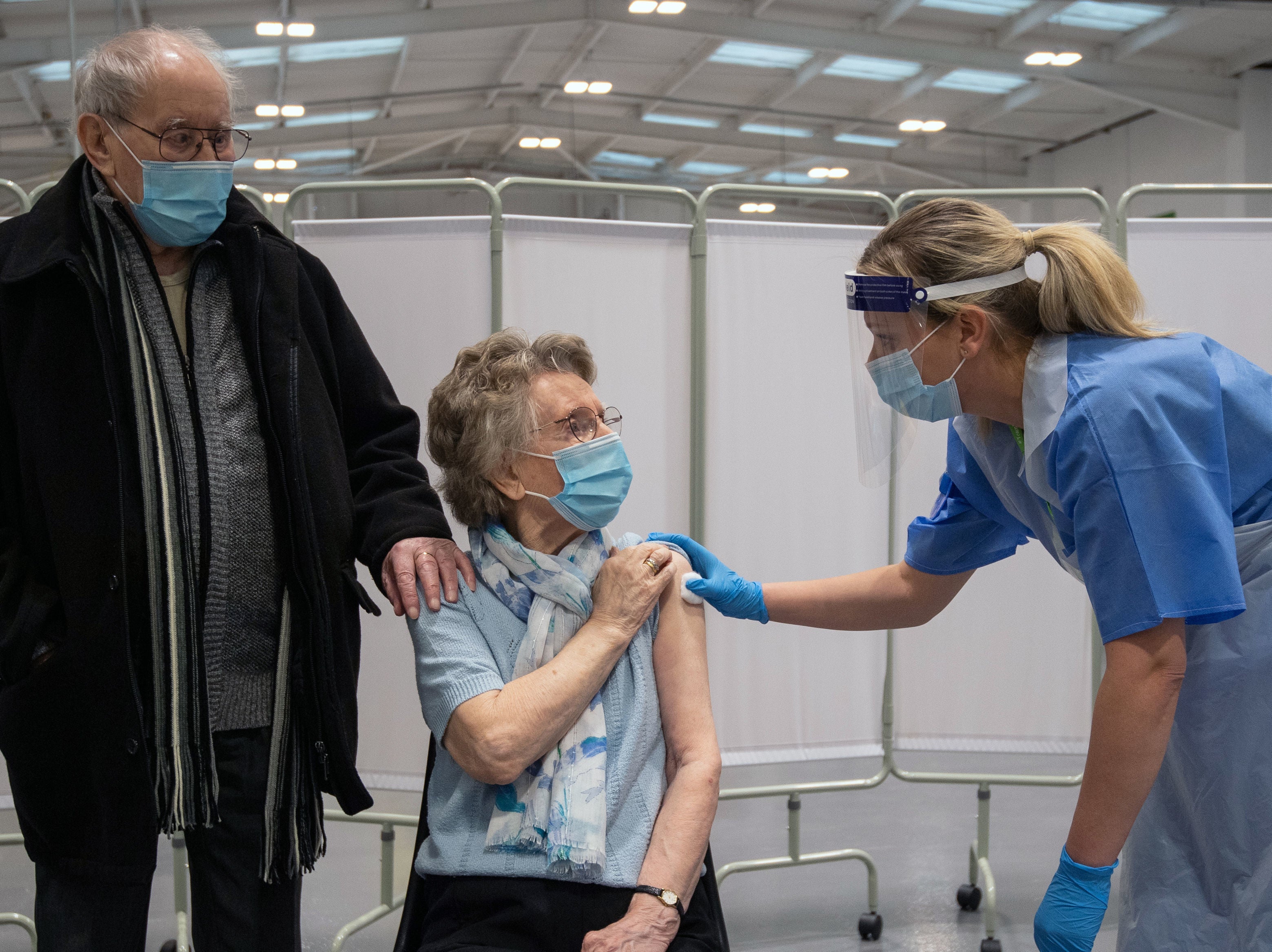
Your support makes all the difference.The EU has backed down over its controversial plan to use a Brexit clause to stop Covid vaccines crossing the Irish border amid the ongoing row over jab supplies.
Downing Street had demanded an urgent explanation after the European Commission said it would trigger Article 16 of the Northern Ireland Protocol to ensure all citizens of the bloc have access to jabs and maintain transparency.
However the move was widely condemned across the UK and Ireland - with both Sinn Fein and the Democratic Unionist Party describing the move as “totally ill judged” and '"an incredible act of hostility".
The Commission later confirmed that it would not trigger Article 16 but warned that it would consider taking action if attempts were made to circumvent export controls imposed on vaccines produced within the bloc. The dispute began after AstraZeneca announced it would reduce its initial supplies of vaccines to the EU by 60 per cent.
It came as the European Medicines Agency granted authorisation for the AstraZeneca coronavirus vaccine for use in adults throughout the EU on Friday.
Meanwhile, a fourth Covid-19 vaccine could be approved for use in the UK in a matter of weeks after clinical trials showed the Novavax candidate was 89 per cent effective in preventing coronavirus.
The Novavax jab, which will be produced on Teesside, will now be assessed by the Medicines and Healthcare products Regulatory Agency (MHRA). The UK has secured 60 million doses of the vaccine, which is believed to offer protection against emerging variants, such as the new UK and South African strains.
- Tony Blair calls for world to come together to fight Covid
- EU steps up threat to block vaccine exports to UK by exploring ‘all legal means’ to keep supplies
- Dubai joins ‘red list’ with travel ban from UAE
- Does the new coronavirus strain have the same symptoms as the first?
- The vaccine bust-up between Britain and the EU is a lose-lose situation - but is it a sign of things to come?
Single-dose Johnson & Johnson vaccine provides strong protection against Covid - study
According to Johnson & Johnson’s large scale trial results, its one-shot vaccine has been shown to generate high levels of protection against Covid-19.
The study, which involved more than 44,000 participants, suggested that the jab was 66 per cent effective in preventing moderate to severe cases of Covid-19. No hospitalisations or deaths were recorded among volunteers.
My colleague Samuel Lovett has the full story:

Single-dose vaccine provides strong protection against Covid, trial finds
The one-shot vaccine manufactured by Johnson & Johnson has been shown to generate high levels of protection against Covid-19, according to the company’s large-scale trial results.
Thousands of travellers stranded with sudden ban on UAE flights
Thousands of travellers are trying to find alternative routes home after the government’s sudden decision to add the UAE to the so-called “red list” of high-risk countries.
All passenger flights from UAE are banned from 1pm today. British residents have been told to find indirect routes home, and will be told to remain in their home with other members of their household for 10 days upon arrival.
Simon Calder reports:

Thousands of travellers stranded with sudden ban on UAE flights
The final arrival from Dubai until further notice is due to arrive at Heathrow just four minutes before the government’s 1pm deadline
Wales schools could reopen after February half-term as cases fall
First minister Mark Drakeford has said Wales will hope to “take advantage” of its lower rate of coronavirus transmissions to get pupils back into schools ahead of other UK nations.
Read the breaking news report below:

Wales schools could reopen after February half-term, Drakeford announces
Wales will hope to "take advantage" of its lower rate of coronavirus transmissions to get pupils back into schools ahead of other UK nations, First Minister Mark Drakeford has said.
North West ambulance service requests army help as Covid calls continue to surge
One of England’s largest ambulance services has requested help from the army to cope with on ongoing surge in coronavirus calls and high staff absence rates.
The North West Ambulance Service has asked for up to 120 troops to be deployed driving vehicles and dealing with patients, reports my colleague Colin Drury.
Get the full story below:

Ambulance service requests army help as Covid calls surge
Troops needed to drive vehicles and deal with patients as pressure continues to mount in north west
Covid timeline: Key dates ahead in vaccine rollout and end of lockdown
When and how lockdown measures will be eased depends on a number of factors, including all of the most vulnerable being vaccinated by 15 February and the effectiveness of those jabs, writes my colleague Chiara Giordano.
She takes a closer look at key dates ahead of the vaccine rollout and the end of the current lockdown in England:
Key dates ahead in vaccine rollout and end of lockdown
Government has set target of vaccinating priority groups by 15 February ahead of lockdown review on same day
Novartis signs initial agreement to help produce Pfizer vaccine
Swiss drugmaker Novartis has signed an initial agreement to help provide manufacturing capacity for the Pfzer/BioNTech coronavirus vaccine.
The move will help boost production amid a blazing row in the EU over vaccine supplies.
Novartis said in a statement: “The agreement will see Novartis utilizing its aseptic manufacturing facilities at its site in Stein, Switzerland.
“Subject to reaching a final agreement, Novartis plans to commence production in the second quarter of 2021.”
AstraZeneca to publish redacted version of EU contract
AstraZeneca will publish a redacted version of its contract with the European Union amid a deepening row between the two sides over vaccine supply shortages in the bloc.
Eric Mamer, chief spokesmen of the European Commission, told a Brussels briefing: "AstraZeneca has agreed to publish the redacted contract signed between the two parties on August 27 2020.
"We welcome the company's commitment towards more transparency in its participation to the rollout of the EU vaccine strategy,” he added.
"Transparency, and accountability, are important to help build trust of European citizens and to make sure they can rely on the effectiveness and safety of the vaccines purchased at EU level."
It comes after EU Commission head Ursula von der Leyen said the contract contains binding orders, as the bloc continue to pressure the drugmaker to deliver supplies as promised.
Ms von der Leyen told Deutschlandfunk radio: “There are binding orders and the contract is crystal clear.
“AstraZeneca has also explicitly assured us in this contract that no other obligations would prevent the contract from being fulfilled.”
Her comments contradicted statements made by the drug company’s chief executive Pascal Soriot earlier this week, as he told newspapers the EU contract was based on a “best-effort” clause and did not commit AstraZeneca to a specific timetable for deliveries.
Former vaccines chief says we need to move from inefficient double jab to pills or sprays
The former head of the UK’s vaccine taskforce has said there needs to be a more efficient way of administering inoculations, including the use of pills, patches of nasal sprays to speed up the process.
As countries grapple with immunising populations against coronavirus, Kate Bingham added that Britain and the EU needed to collaborate to produce “tweaked” vaccines against new variants that may arise, reports our Political Correspondent Ashley Cowburn.
Read the story below:
Former UK vaccines chief says we need to move from inefficient double-jab to pills or sprays
UK needs to work with EU to develop ‘tweaked’ vaccines to new variants, says Kate Bingham
Op-ed: Government must reveal strategy for removing travel restrictions
“The government cannot continue to crush everyone’s hopes and dreams without revealing a strategy for removing the tangle of restrictions it is creating,” says The Independent’s travel correspondent.
Read Simon Calder’s op-ed below:

Even amid the continuing tragedy, we cannot have all our travel dreams crushed
The Man Who Pays His Way: The world will soon start to open up. Where is the UK’s plan to do the same?
Oxford vaccine ‘absolutely safe and effective’, says JCVI chief after German ruling
Professor Anthony Harnden, deputy chair of the Joint Committee on Vaccination and Immunisation (JCVI), has stressed that the Oxford/AstraZeneca vaccine is “absolutely safe and effective”.
It comes after Germany recommended the AstraZeneca vaccine only be given to people under the age of 65. The country’s vaccine committee said there was a lack of sufficient data on the effectiveness of the jab on older people.
But Prof Harnden said Germany had far more Pfizer jabs than AstraZeneca jabs, and wanted to prioritise its larger supply for its elderly population.
He told BBC Radio 4's Today programme: "This vaccine is incredibly effective - the argument is not about effectiveness actually, it is about precision of size of effect.
"When the German advisory body which looked at this data, they had in mind - like we did in JCVI - the whole picture.
"And actually they don't have very much supply of Oxford/AstraZeneca at the moment, they've got lots of supply of Pfizer.
"And so they said that, given the limited precision of estimate for the Oxford data, that they would prefer to use the majority of their Pfizer vaccine in the older people."

Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments