DeepMind research predicts structure of almost every known protein
The breakthrough will help to tackle major global challenges such as developing malaria vaccines and fighting plastic pollution.
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
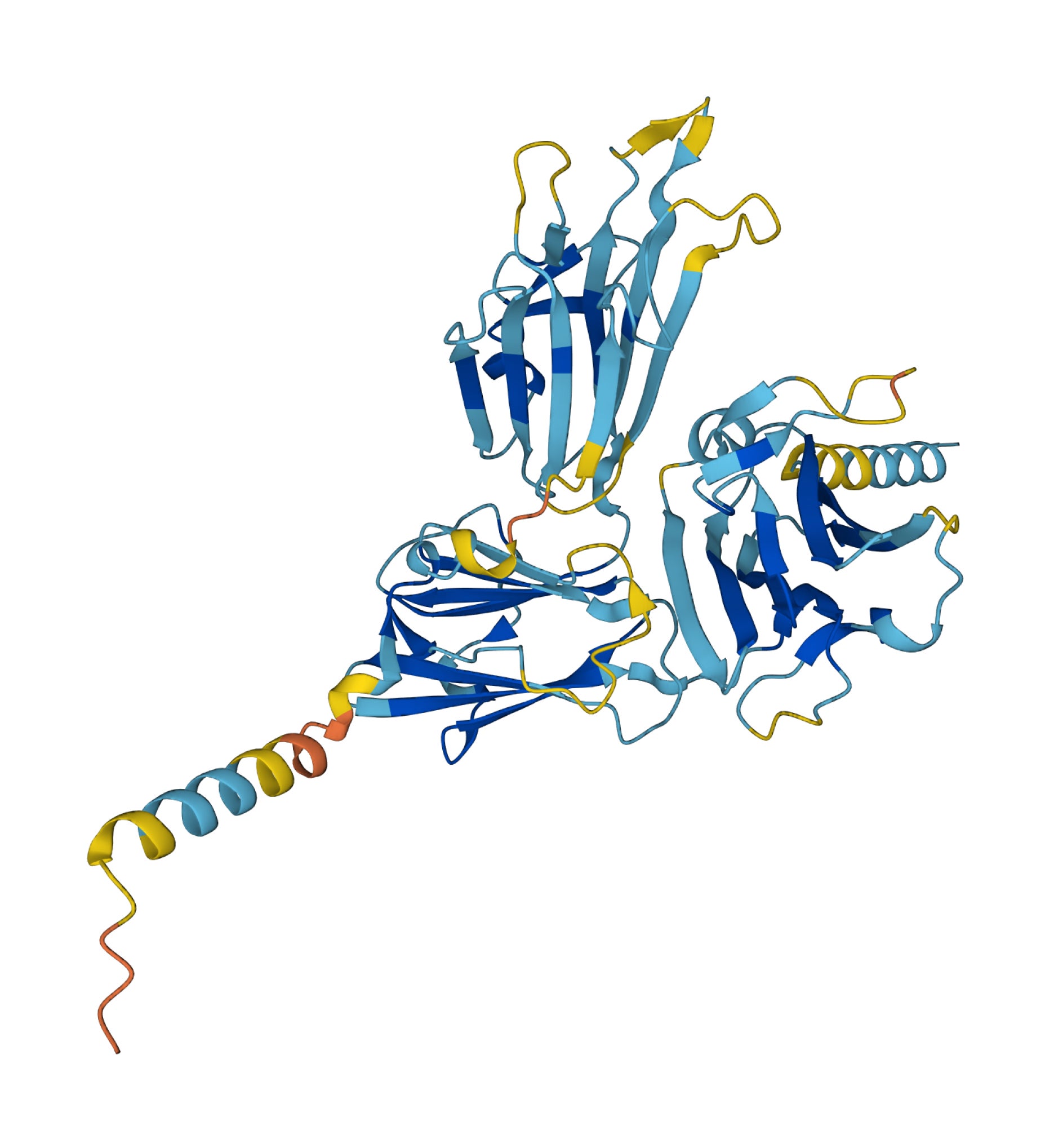
Your support makes all the difference.Artificial intelligence has mapped out the shape of virtually every protein known to science.
The breakthrough will help to tackle major global challenges such as developing malaria vaccines and fighting plastic pollution, experts say.
Proteins are the building blocks of life, and their shape is closely linked to their function.
Being able to predict a protein’s structure gives scientists a better understanding of what it does and how it works.
Our hope is that this expanded database will aid countless more scientists in their important work and open up completely new avenues of scientific discovery
The research was conducted by DeepMind and EMBL’s European Bioinformatics Institute (EMBL-EBI), which used the AlphaFold AI system to predict a protein’s 3D structure.
The AlphaFold Protein Structure Database – which is freely available to the scientific community – has been expanded from nearly one million protein structures to more than 200 million structures, covering almost every organism on Earth that has had its genome sequenced.
The expansion includes predicted shapes for the widest possible range of species, including plants, bacteria, animals, and other organisms, opening up new avenues of research across the life sciences.
Demis Hassabis, founder and CEO of DeepMind, said: “We’ve been amazed by the rate at which AlphaFold has already become an essential tool for hundreds of thousands of scientists in labs and universities across the world.
“From fighting disease to tackling plastic pollution, AlphaFold has already enabled incredible impact on some of our biggest global challenges.
“Our hope is that this expanded database will aid countless more scientists in their important work and open up completely new avenues of scientific discovery.”
In December 2020, AlphaFold was recognised as a solution to the 50-year-old grand challenge of protein structure prediction by the organisers of the Critical Assessment of protein Structure Prediction (Casp).
At the time, it demonstrated that it could accurately predict the shape of a protein, at scale and in minutes, to atomic accuracy.
The database works like an internet search for protein structures by providing instant access to predicted models.
This cuts down the time it takes for scientists to learn more about the likely shapes of the proteins they are researching, speeding up experimental work.
Earlier predictions have already helped scientists in their quest to create an effective malaria vaccine.
Scientists at the University of Oxford and the National Institute of Allergy and Infectious Diseases have been researching a protein called Pfs48/45, which is one of the most promising candidates for inclusion in a transmission-blocking malaria vaccine.
Existing technology alone did not allow them to fully understand the structure of the protein in order to see where the most effective transmission-blocking antibodies bind across its surface.
Matthew Higgins, professor of Molecular Parasitology and co-author of that study, said: “By combining AlphaFold models with our experimental information from crystallography, we could reveal the structure of Pfs48/45, understand its dynamics and show where transmission-blocking antibodies bind.
“This insight will now be used to design improved vaccines which induce the most potent transmission-blocking antibodies.”
DeepMind and EMBL-EBI said they will continue to refresh the database periodically, with the aim of improving features and functionality.