Students taking part in clinical trials: What you need to know
You've seen the flyers around campus. Maybe you've wondered what being a medical guinea pig is like? Tom Clarke finds out more
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.Students around the country are feeling the pinch when it comes to their living costs. Many are choosing to take the strain off the bank of Mum and Dad by supplementing their maintenance loans through alternative income sources.
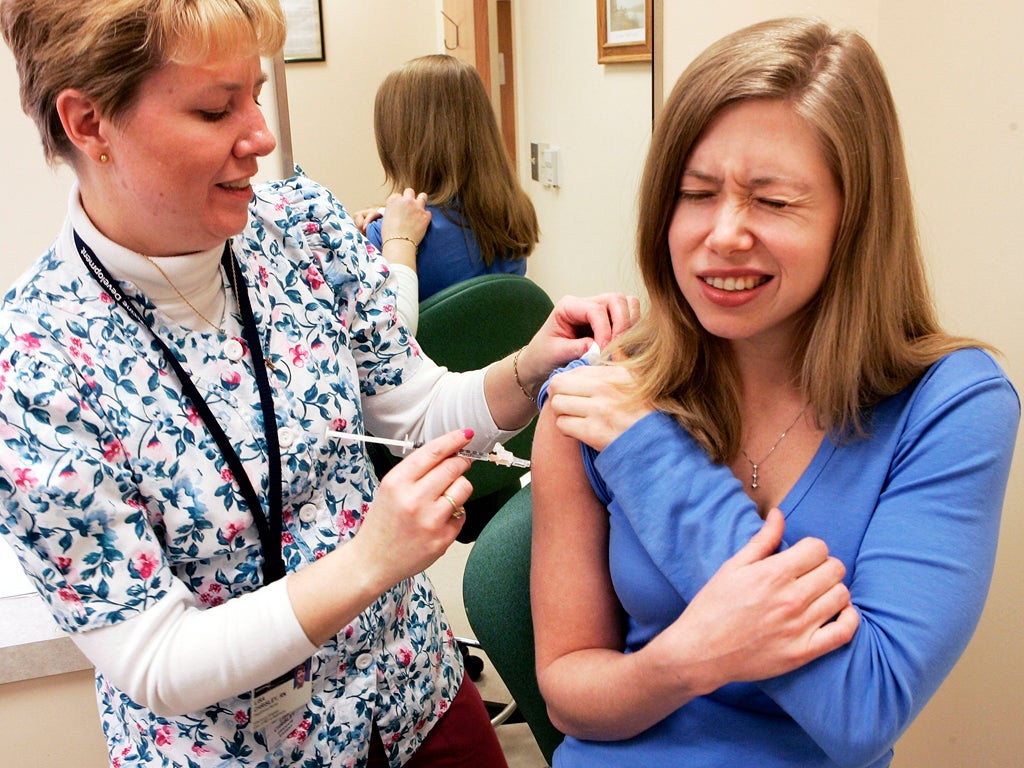
Here's one: pharmaceutical companies are crying out for volunteers, given that around one quarter of paid UK clinical trials finish early because of a lack of suitable participants.
When people are asked whether they would volunteer for a clinical trial they seem to fall into two camps. On one side, some consider medical tests to be fraught and reckless, and on the other, are those who see the prospect of earning £4,000 in a 30-day trial as easy money. The reality of life as a test subject, in the majority of cases, is far removed from both.
What to expect
If you are volunteering in a phase 1 clinical trial - which means the drug is being trialled for the first time on human patients - then you can expect daily rates of around £120. However for clinical trials it’s not as simple as turning up on the day and handing over a few bodily fluids while you pop a couple of pills.
There is an extensive audition process to see if individuals are right for the trial. James Merryland*, a 22-year-old law student, recently volunteered for a phase 1 clinical trial that was looking to test a drug that reduces inflammation on the lungs. This week trial involved two three-hour pre-screening sessions, four days in a clinic and a three-hour follow-up. For this, James received £1,100 plus food and board when staying at the clinic.
He underwent a thorough medical examination that included a physician taking his BMI, weight, blood, urine, heart rate and V02 max. After passing this initial assessment, James signed documents stating he understood what he was volunteering for and accepted the marginal risks involved.
“They told me there might be no side effects," he said, "but that I might feel slightly lethargic for a few days or develop mild flu like symptoms.”
Duty of care
This does not mean that the company did not have a duty of care towards James and his safety. The MHRA (Medicines and Healthcare Products Regulatory Agency) screens all drugs that are put forward for clinical trial. All clinical trials are also put before an Independent Ethics Committee to ensure it is in the interests of the public and medical profession. The rights of the subjects are also protected by an independent review board (IRB). The number one priority of IRBs is to protect human subjects from serious physical or psychological harm.
Dr Ben Goldacre, research fellow in epidemiology at the London School of Hygiene and Tropical medicine, believes that one of the fears people have is that they might be given some new and elaborate experimental drug'.
"Firstly, that is very uncommon, and secondly, before you start the trial people will explain very clearly what you are getting. There are some disadvantages to participating in a clinical trial and most of those revolve around inconvenience.”
In separate trial, Emily Jones*, a 23-year-old history student, was flown to Mexico and provided with top-level medical insurance, a week’s accommodation and an emergency phone while she tested a drugs patch to stop traveller’s diarrhoea. Emily duly received 'much better medical insurance' than she otherwise would have.
She was expected to attend three check-ups at specified places in Mexico and Guatemala while on a six-week trip she had organised with friends. Overall Emily had to wear the patch for a total of two months. Not only was the majority of the trip paid for but the patch worked for Emily as she didn’t get ill.
“The experience has definitely made me think about volunteering for trials again in the future,” she said.
Inconvenience
Both James and Emily cited the real difficulty with volunteering with these trials was the inconvenience caused by having to attend repeated check-ups, rather than the risk posed by the drugs themselves.
“Whilst in clinic I was told to meet certain requirements as far as the food I ate and at what time I ate it,” said James, who was also prohibited from drinking alcohol for the week, and expected to use two methods of contraception for three months after the trial to negate the risk of any side effects being past to a third party via bodily fluids.
“If you failed to meet any of these requirements, I was told my payment would be dramatically reduced.”
Despite the risk and the strict rules over his lifestyle for the week, James enjoyed the experience, he said, “I’m in the middle of revising for my final exams of my law degree, I spent any downtime I had at the clinic revising, except this time I was getting paid for it!”
There is always risk and it is always best to know exactly what the short-term and long-term consequences of the trial, yet it seems the real trying aspect of any extended trial is the inconvenience that it brings as far as attending repeated meetings and examinations. If you can stomach this, as well as the pills, then the financial reward and knowledge that you’re contributing to medical science may make you seriously consider clinical trials as a viable way of supplementing a student income.
* Names have been changed
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments