Shops advised to remove Genrui-branded antigen tests from shelves
The Health Products Regulatory Authority said they should not be used, pending further investigation, after over 550 complaints of false positives.
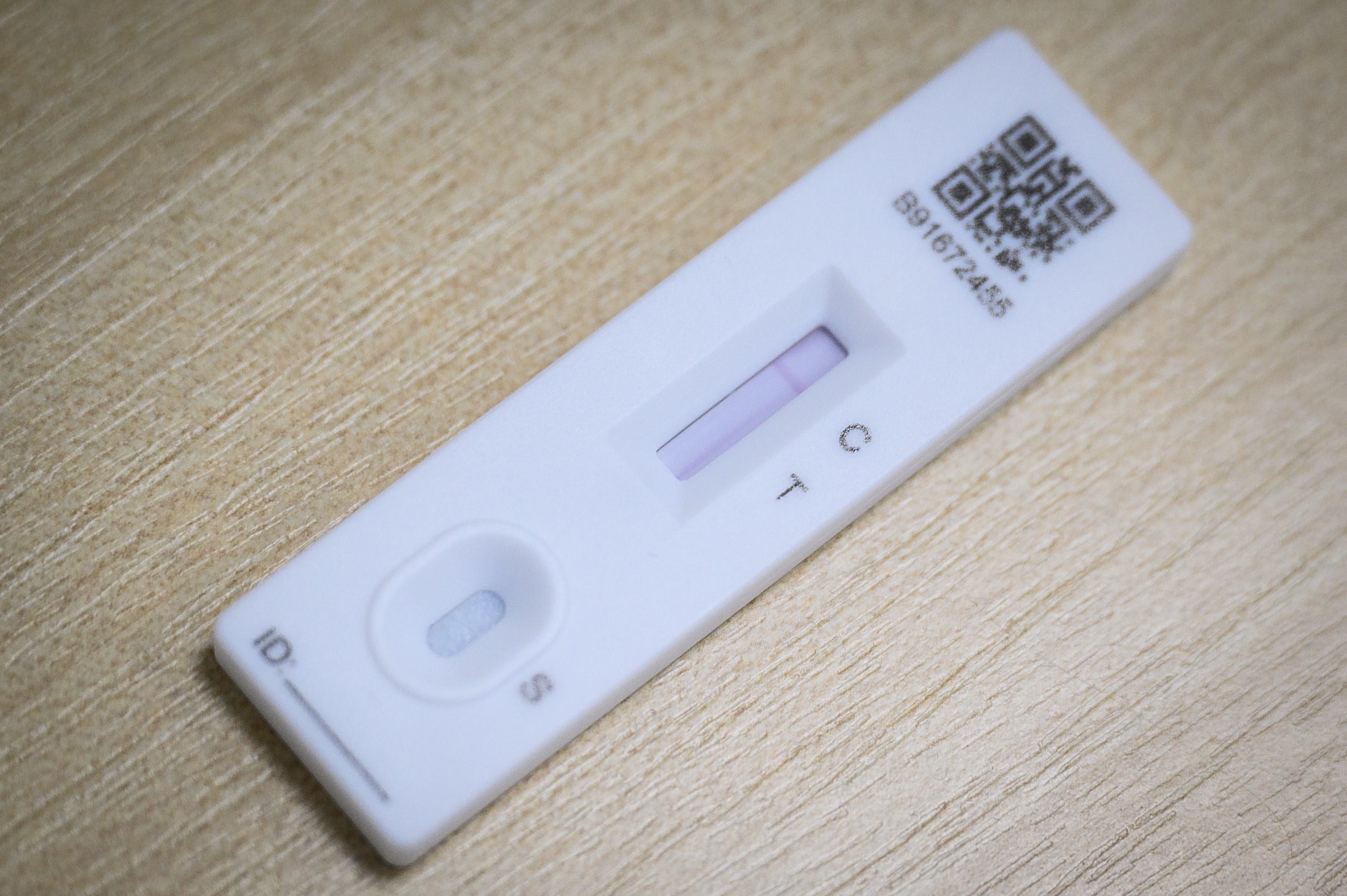
Retailers have been advised to remove “Genrui” branded antigen tests from their shelves by the Health Products Regulatory Authority.
The HPRA has asked outlets to take the voluntary measure pending a further investigation, after it was flooded with more than 550 complaints of false positive results associated with the brand of Covid-19 test.
While all rapid antigen tests have the potential to provide false positive results, the HPRA said the “rapid rise” in reports linked to Genrui was significant and meant a “precautionary removal from sale” is warranted.
The matter is set to be further investigated by the manufacturer Genrui Biotech, based in Shenzen, China and its European representative.
The HPRA also advises against the online purchase of Genrui self-tests at this time while the matter remains under investigation.
“Individuals who have received a positive result following use of any rapid antigen self-test, including this test, should follow the current public heath advice on the HSE website” the HPRA said in a statement.
“The HPRA will continue to liaise with the manufacturer to investigate the matter further, and is also in contact with other European Competent Authorities in relation to this issue.
“Individuals who have experienced a false positive or false negative result can report the occurrence via the HPRA website – www.hpra.ie
“The HPRA wishes to acknowledge the cooperation and swift response of retailers in relation to this matter.
“Any retailers supplying this product who have not yet received communication in respect of this issue should contact devicesafety@hpra.ie.”
Bookmark popover
Removed from bookmarks