US allows sexually inactive gay men to donate blood
Gay rights and AIDS awareness advocates balk at decision they say 'falls short' of acceptable response to three-decade ban
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
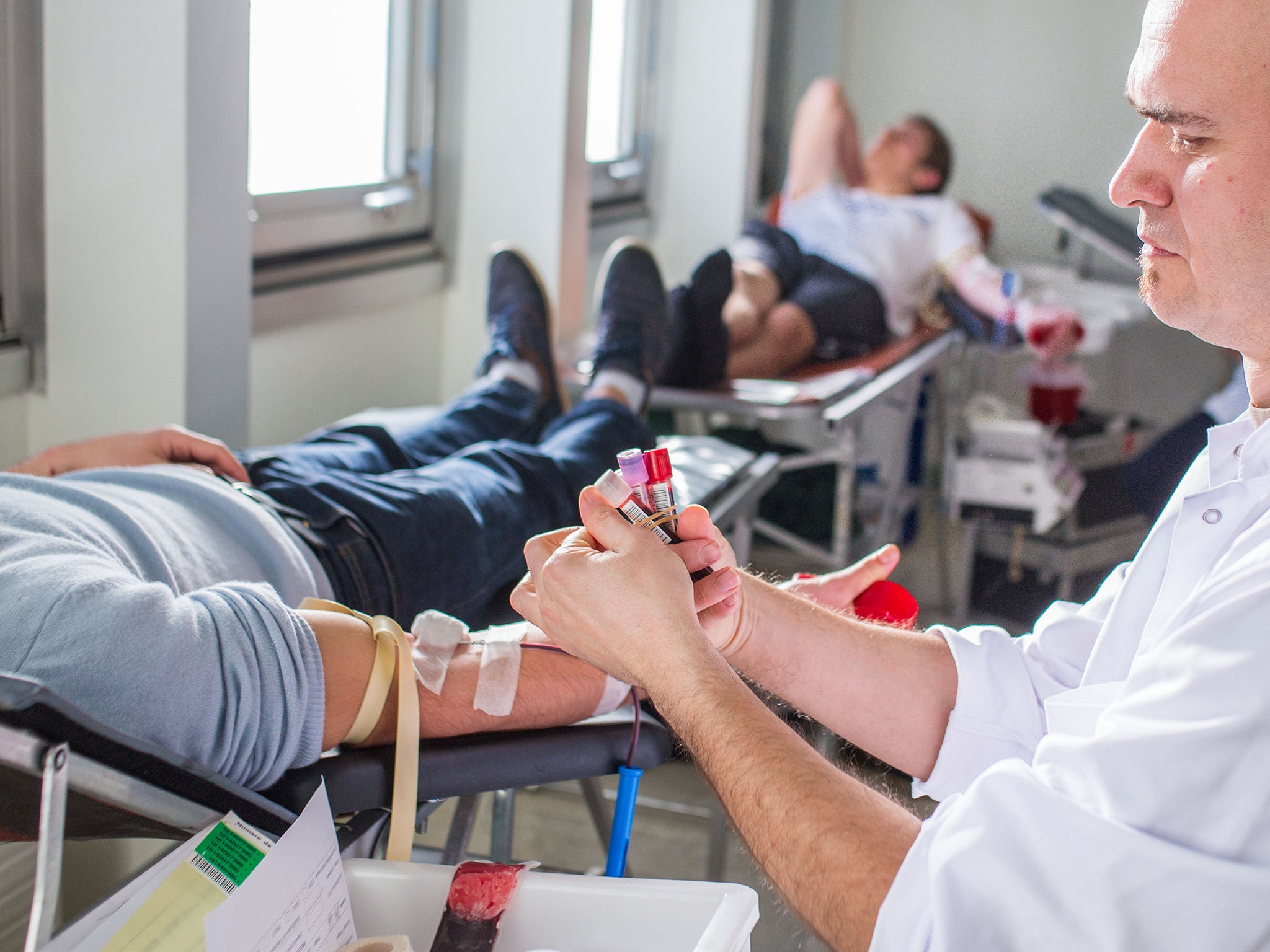
Your support makes all the difference.US health officials on Monday relaxed a three-decade ban on gay men donating blood.
But gay rights advocates, who have long argued that the ban is “medically and scientifically unwarranted,” said the measure “falls far short of an acceptable solution.”
Now, gay men who remain sexually inactive for 12 months are allowed to give blood, the Food and Drug Administration (FDA) announced in a press release.
“Previously, there was not enough scientific evidence to support a change in blood donation policy for men who have sex with men in the United States,” FDA spokeswoman Tara Goodin told The Independent. “Based on careful consideration of recently completed studies and epidemiological data, the FDA has concluded that the blood donation policy can be changed from an indefinite deferral to a 12-month deferral, without compromising the safety of the blood supply.”
Gay rights advocates balked at the ruling.
“This new policy prevents men from donating life-saving blood based solely on their sexual orientation rather than actual risk to the blood supply,” the government affairs director at the Washington-based Human Rights Campaign (HRC), David Stacy, said in a press release.
"While it's a step in the right direction toward an ideal policy that reflects the best scientific research, it still falls far short of a fully acceptable solution because it continues to stigmatize gay and bisexual men,” Stacy added.
AIDS awareness and health care advocates joined Stacy in condemning the FDA decision.
“Given how far we’ve advanced in the methods for detecting HIV, this FDA decision signals that the time has passed for blood donation policies that are based more on fear than science,” Christopher Johnson, spokesman for the Los Angeles-headquartered AIDS Healthcare Foundation told The Independent. “Regulations that prohibit a whole class of people from donating blood when the need is so great should definitely be revised.”
HRC added that The American Red Cross, America's Blood Centers, and the American Association of Blood Banks have characterized the blood ban as medically and scientifically unwarranted as far back as 2006. But the organisations issued a joint press release commending the FDA decision, saying the "policy change aligns the MSM donor deferral period with those for other activities that may pose a similar risk of transfusion-transmissible infections."
The FDA’s Ms Goodin responded to the advocacy groups, saying, “The deferral policy is a behavior-based policy, not one based upon sexual orientation.”
“Current epidemiology shows that a history of male-to-male sexual contact was associated with a 62-fold increased risk for being HIV positive, whereas the increase in risk for a history of multiple sexual partners of the opposite sex in the last year was 2.3-fold," Ms Goodin said. "The FDA carefully considered alternative deferral criteria, such as individual risk assessment for individual risk of HIV, as alternatives to a time-based deferral. However, evidence shows that self-reporting presents significant issues in the U.S. for a number of reasons, including lack of sufficient data on the effectiveness of donor educational questionnaires and lack of reliability in self-reports of monogamy by partners in any type of sexual relationship. On the other hand, a 12-month deferral has been well studied and found to maintain the safety of the blood supply in Australia, a country with HIV epidemiology and blood screening systems similar to the United States.”
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments