Fourth person dies in US from bacteria linked to recalled eye drops
Fourteen individuals have gone blind and four others had their eyes surgically removed
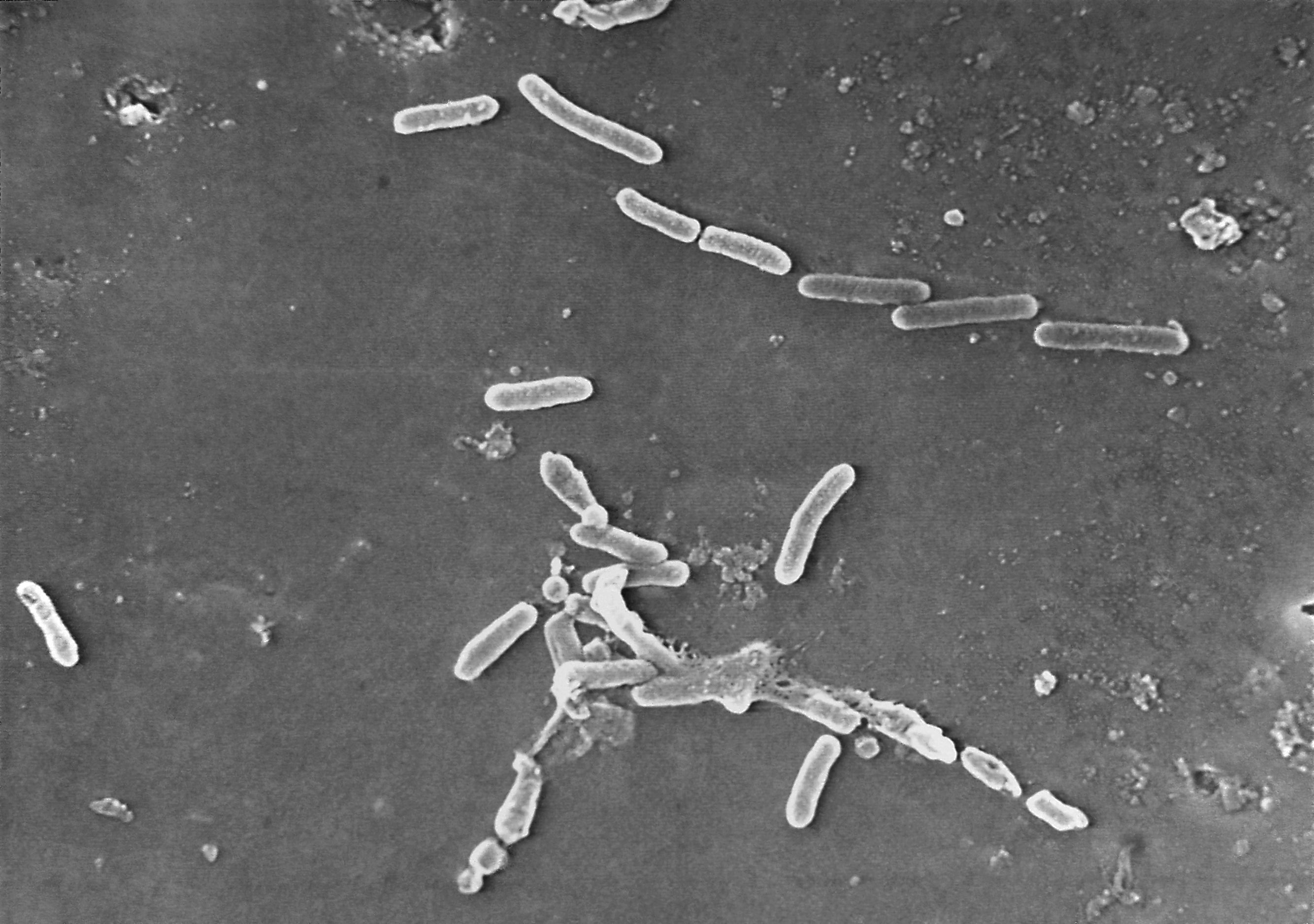
A fourth person in the US has died from exposure to a drug-resistant bacteria found in a now-recalled type of eye drops.
Eighty-one patients in 18 states have been affected by the bacteria after using EzriCare or Delsam Pharma’s Artificial Tears products, the Centers for Disease Control and Prevention said in its most recent update.
More than 10 brands of artificial tears have been recalled due to the presence of the bacteria of Pseudomonas aeruginosa, which is resistant to standard antibiotics.
The contaminated products, made by the India-based company Pharma Healthcare, were recalled in February, with at least seven patients diagnosed afterward.
In addition to the four deaths, 14 individuals have gone blind and four others had their eyes surgically removed.
A visit by US health inspectors to the factory uncovered failures to maintain sterility.
According to the Food and Drug Administration, the plant used “a deficient manufacturing process” between December 2020 and April 2022 for products shipped to the US.
“Testing of opened product identified the outbreak strain in bottles of EzriCare Artificial Tears that were obtained from two states,” the CDC previously told ABC News in a statement. “Testing of unopened product to evaluate for intrinsic contamination is ongoing by [the U.S. Food and Drug Administration].”
The CDC has warned anyone who has used the products recently to seek medical care immediately.
The eighteen states where cases have been reported are: California, Colorado, Connecticut, Delaware, Florida, Illinois, North Carolina, New Jersey, New Mexico, Nevada, New York, Ohio, Pennsylvania, South Dakota, Texas, Utah, Washington and Wisconsin.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments


Bookmark popover
Removed from bookmarks