A blinding bacteria, four dead and an everyday brand blamed: What we know about the US eye drop recall
People have had to have their eyes surgically removed due to the bacteria
Four people have died and others have gone blind and even had their eyes surgically removed after being exposed to a drug-resistant bacteria found in a now-recalled type of eyedrops.
The US Centres for Disease Control and Prevention released an update on Friday (19 May), alerting the public to a rise in deaths and injuries caused by the bacteria.
Here's everything we know about the recalled eyedrops and the deadly bacteria.
Contaminated eyedrops
More than 10 brands of artificial tears have been recalled due to the presence of the bacteria, according to ABC News.
Eighty-one patients in 18 states have been affected by the bacteria after using EzriCare or Delsam Pharma’s Artificial Tears products, the CDC said in its most recent update.
In addition to the four deaths, fourteen individuals have gone blind and four others had their eyes surgically removed.
The contaminated products, made by the India-based company Pharma Healthcare, were recalled in February, with at least seven patients diagnosed afterwards.
The eye drops were contaminated with a drug-resistant form of Pseudomonas aeruginosa, a particularly aggressive bacterium, according to the CDC.
A visit by US health inspectors to the factory uncovered failures to maintain sterility.
According to the Food and Drug Administration, the plant used “a deficient manufacturing process” between December 2020 and April 2022 for products shipped to the US.
"Testing of opened product identified the outbreak strain in bottles of EzriCare Artificial Tears that were obtained from two states," the CDC told ABC News in a statement. "Testing of unopened product to evaluate for intrinsic contamination is ongoing by [the U.S. Food and Drug Administration]."
The FDA issued a warning last month urging the public not to buy the companies' Artificial Tears due to the potential contaminants. The CDC has warned anyone who has used the products recently to seek medical care immediately.
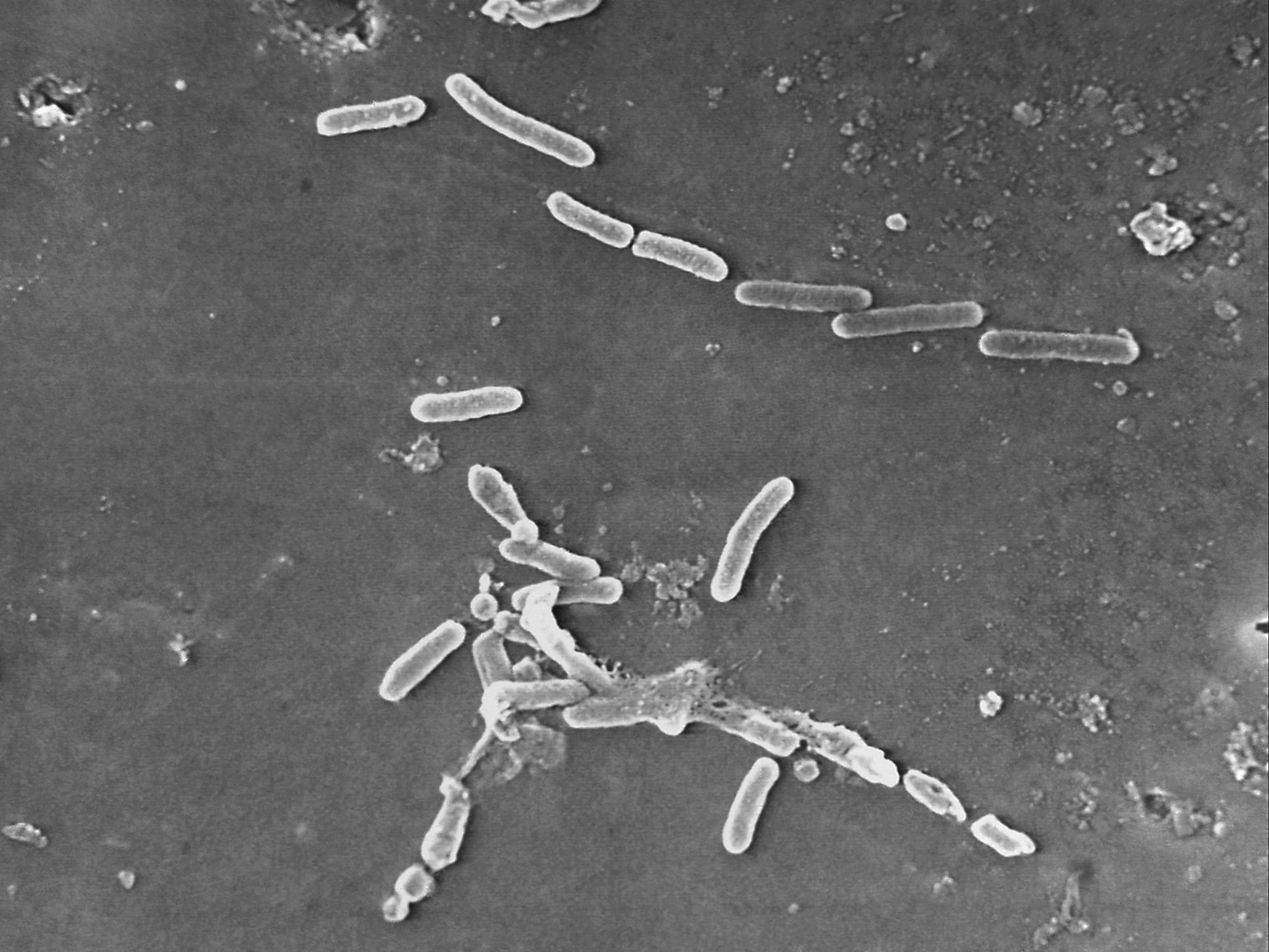
Pseudomonas aeruginosa bacteria
The bacteria is found in the environment, and the aeruginosa type is the most common to cause infections in humans.
The infection most commonly spreads in hospitals and other health care settings, and is generally transferred between unclean hands, equipment, or improperly cleaned equipment.
The bacteria is resistant to numerous antibiotics and has caused approximately 32,000 infections among hospitalised patients in the US. It is also responsible for approximately 2,700 deaths, according to the CDC.
The strain linked to the recent outbreak has never been reported in the US before, according to the agency.
US Cases
Three of the fourth deaths caused by the bacteria have occurred in Washington state.
The other states where cases have been reported are: California, Colorado, Connecticut, Delaware, Florida, Illinois, North Carolina, New Jersey, New Mexico, Nevada, New York, Ohio, Pennsylvania, South Dakota, Texas, Utah, Washington and Wisconsin.
One of the newest case reports described a 72-year-old woman who lost her vision in her left eye after using the ExriCare product for approximately a week, according to CNN.
She started noticing some blurry vision in her left eye for a few days,” Dr. Ahmed Omar, an ophthalmologist at University Hospitals Cleveland Medical Center, who treated the woman, said. “It was initially painless, but according to the patient and her husband, one morning she woke up and she had a yellow discharge on her pillow. And that’s when she started noticing that the appearance of her eye had changed.”
The woman had to be admitted to the emergency room, where physicians discovered a large ulcer on her left cornea.
In the most extreme cases, the infection can spread to other body parts from the eyes, including the bloodstream, respiratory tract, urinary tract, and and the cornea.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments

Bookmark popover
Removed from bookmarks