Popular anxiety drug recalled over potentially ‘life-threatening’ issue
The US Food and Drug Administration said taking wrong dose of Clonazepam could result in ‘significant sedation, confusion, dizziness, diminished reflexes, ataxia, and hypotonia’
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.The US Food and Drug Administration has recalled the anti-anxiety drug Clonazepam due to a potentially "life-threatening" labeling issue, according to the agency.
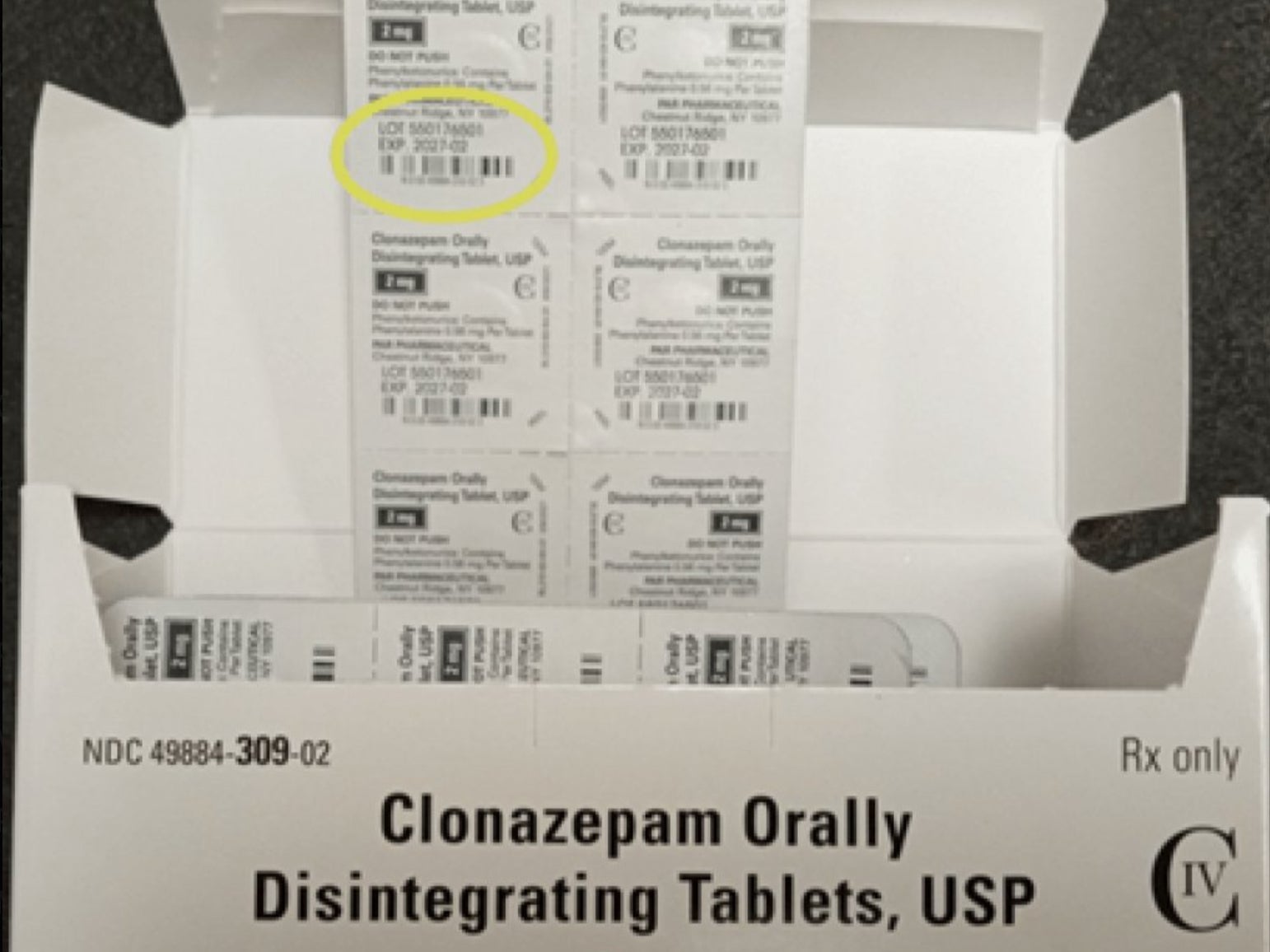
The FDA said in a statement that Endo Inc., a pharmaceutical provider, issued a voluntary recall of 16 lots of Clonazepam Orally Disintegrating Tablets after it discovered they were mislabeled. The labels listed the wrong dosage strength and included an incorrect National Drug Code on their packaging.
The company said the mislabelling was an error made by a third-party packaging company.
The FDA warned that adults and children taking the drug could face "life-threatening" side effects if they take an incorrect dose of the drug.

The agency said that taking the wrong dosage of the drug could result in "significant sedation, confusion, dizziness, diminished reflexes, ataxia, and hypotonia."
"There is reasonable probability for significant, possibly life-threatening, respiratory depression especially for patients with concomitant pulmonary disease, patients who have prescribed dosing near maximal dosing, and patients also taking other medications that could cause additional respiratory depression," the FDA said in its statement.
Endo Inc. said that, as of November 21, there have been no reported incidents of ill effects caused by the recalled products.
The affected lot numbers can be found here.
Anyone with unused prescribed cartons of the drug that fall under the affected lot numbers has been advised to stop taking the drug.
Retailers have also been asked to immediately stop selling any of the affected lots.
“Distributors, retailers that have the product lot being recalled should immediately stop distributing and dispensing and return to the place of purchase or contact Inmar,” the FDA said.
Consumers who may have taken an incorrect dose for any reason are advised to contact a physician, the FDA said.
Anyone with questions about the recall can call Inmar Inc. — which is handling the recall — at 855-589-1869 or email the company at rxrecalls@inmar.com.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
1Comments