Human embryo models research may pave the way for improved IVF rates – study
The research could also lead to the development of therapeutics for infertility or non-hormonal contraceptives.
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.New research on human embryo models paves the way for improving the success rate of IVF and creating a new non-hormonal, user-friendly contraception, a study suggests.
While hormonal contraceptives have been successfully used for a long time by many women, they can have side-effects, and their efficiency decreases if they are not faithfully taken on a daily basis.
Additionally, some women, such as breast cancer survivors, cannot be subjected to hormonal treatments.
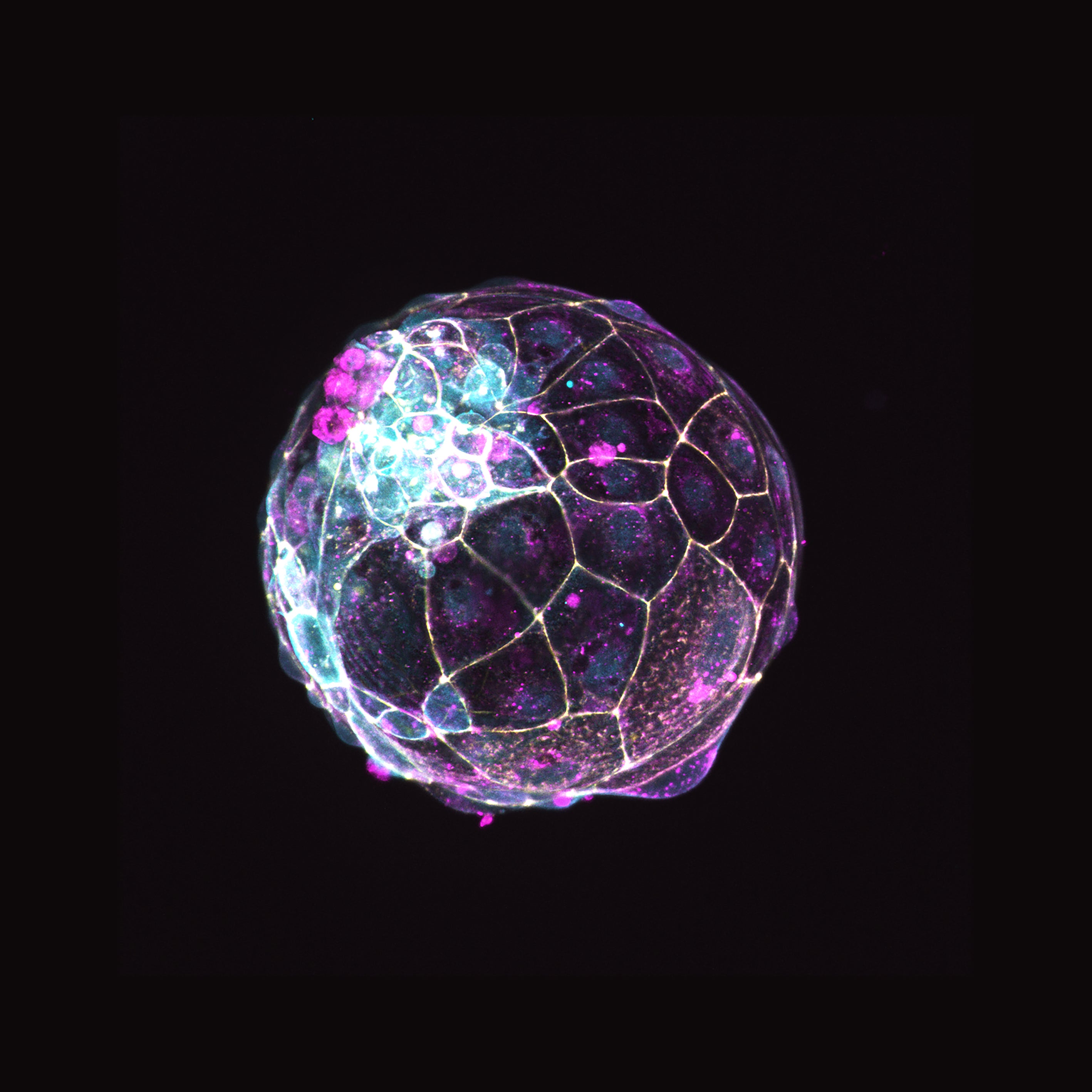
In the new study, human blastoids – structures that mimic the early human embryo – are shown to replicate key phases of early development of the human embryo, including the attachment to cultured uterine cells.
The research could help increase the understanding of the early stages of human development and to develop therapeutics for infertility or contraceptives.
Institute of Molecular Biotechnology (IMBA) of the Austrian Academy of Sciences researcher Nicolas Rivron and his team discovered molecules that could be candidates for contraceptives or fertility enhancers.
Dr Rivron said: “We want to make family planning easier, more convenient, and more adapted to current societal challenges.”
Within a week of fertilisation, human embryos form a ball of cells called a blastocyst, which implants into the uterine wall.
A faithful model of this developmental stage could support research to explore implantation and early development.
Using a SC144 molecule, the researchers found a promising pathway for a new generation of non-hormonal contraceptives.
They suggest these birth control pills could be taken only if necessary, removing the burden and stress associated with medications that need to be taken daily.
The need for only an occasional dose might also result in significantly fewer side effects compared to a daily hormonal pill, the scientists say.
Researchers were able to stimulate human stem cells to efficiently and faithfully self-organise into realistic models of the earliest stages of embryonic development.
These in vitro models, called blastoids, allowed them to begin studying the basic principles of early human development and to search for new therapeutics.
The blastoids were cultured for up to 13 days, at which point they contained about 300 cells.
Beyond contraception, the team also used blastoids to discover a new effect of a natural molecule, LPA.
According to the study, this molecule strongly improves the self-organisation of the stem cells and therefore might be used to boost the formation of natural embryos during In Vitro Fertilisation (IVF) procedures.
Dr Rivron said: “We hope we can use such molecules to improve the number and quality of IVF embryos, and the chance of becoming pregnant.
“Our goal is to empower women by allowing them to better control their fertility, whether they wish to prevent pregnancy or enhance their chances of having a child.”
He added: “By using blastoids we speed up research and make it more ethical.”
Dr Rivron concluded: “It is clear that scientific and biomedical knowledge will skyrocket with such realistic in vitro models for early pregnancy.”
The research is published in the Nature journal.