Government enables more health workers to administer flu and Covid vaccines
Ministers hope changes will help quick role out of potential coronavirus vaccine
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
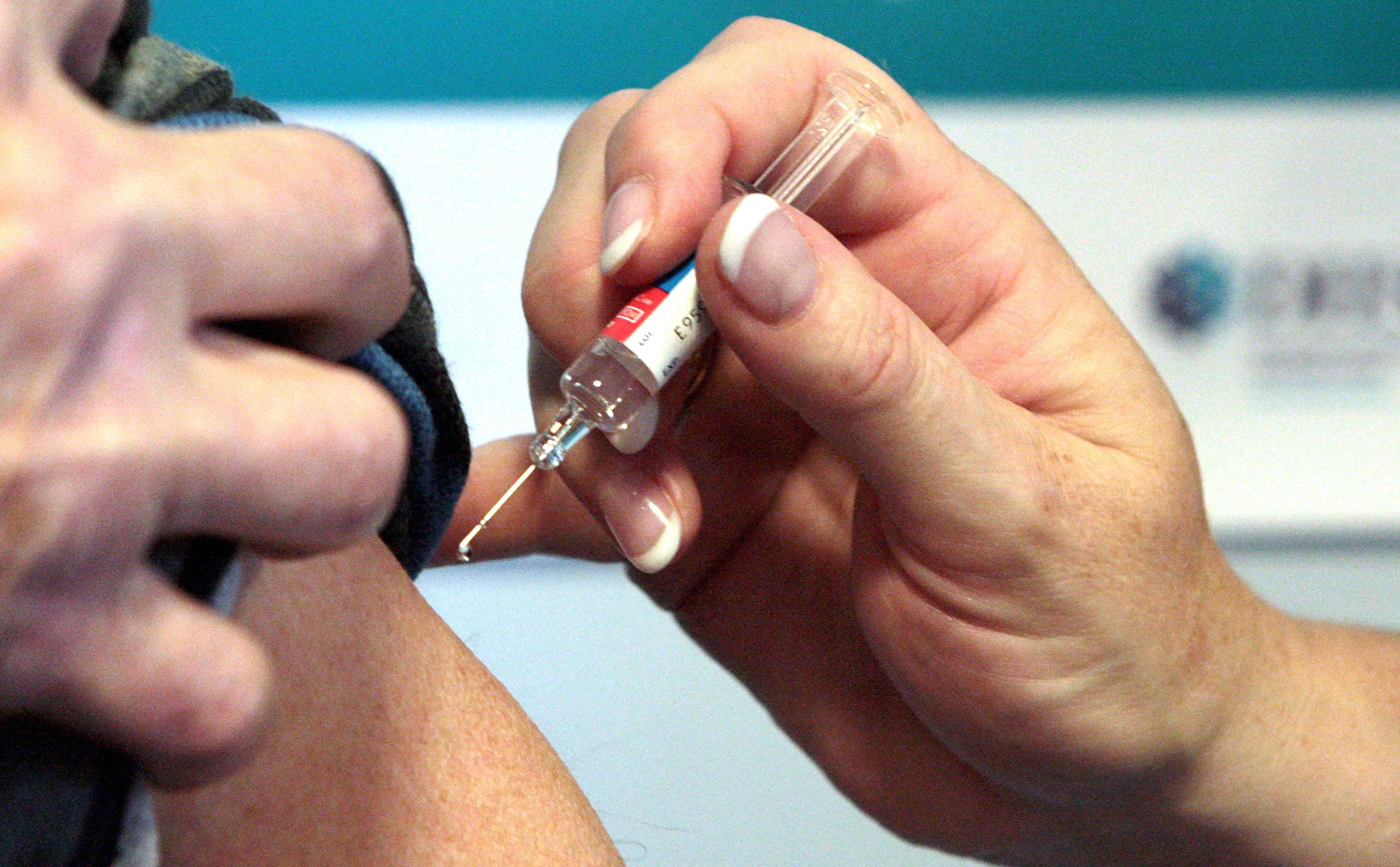
Your support makes all the difference.The government has introduced legal changes that permit more health workers to administer vaccines once they are clinically approved.
The amendments to the Human Medicine Regulations 2012 come into effect on Friday, meaning that experienced workers such as independent nurses, pharmacists and physiotherapists will be able to give vaccinations after undergoing training.
It is hoped that this measure will help the speed at which any potential coronavirus vaccine could be rolled out.
Speaking about the new legislation, health secretary Matt Hancock said: “The NHS has vast experience in vaccinating millions of people against diseases every year.
“These legal changes will help us in doing everything we can to make sure we are ready to roll out a safe and effective Covid-19 vaccine as soon as it has passed clinical trials and undergone rigorous checks by the regulator.”
Mr Hancock added that the new provisions would not affect pre-existing hospital, GP and community services.
Jonathan Van-Tam, England’s deputy chief medical officer, said the changes “aim to improve access and strengthen existing safeguards protecting patients”.
“If a vaccine is developed before 2021, the changes to the Human Medicine Regulations will bolster existing powers that enable the Medicines and Healthcare products Regulatory Agency (MHRA) to authorise temporary supply for any treatment or vaccine needed to respond to a public health need,” he added.
The amendments allow fast-track mass vaccination to take place without the approval of the European Medicines agency, which formerly had to issue a vaccine licence.
As of early September, the UK had pre-ordered 380 million Covid-19 doses, which works out as almost six per person, the highest rate in the world.



Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments