Ovarian cancer blood tests breakthrough: Huge success of new testing method could lead to national screening in Britain
The 14-year trial detected 86% of ovarian cancers
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
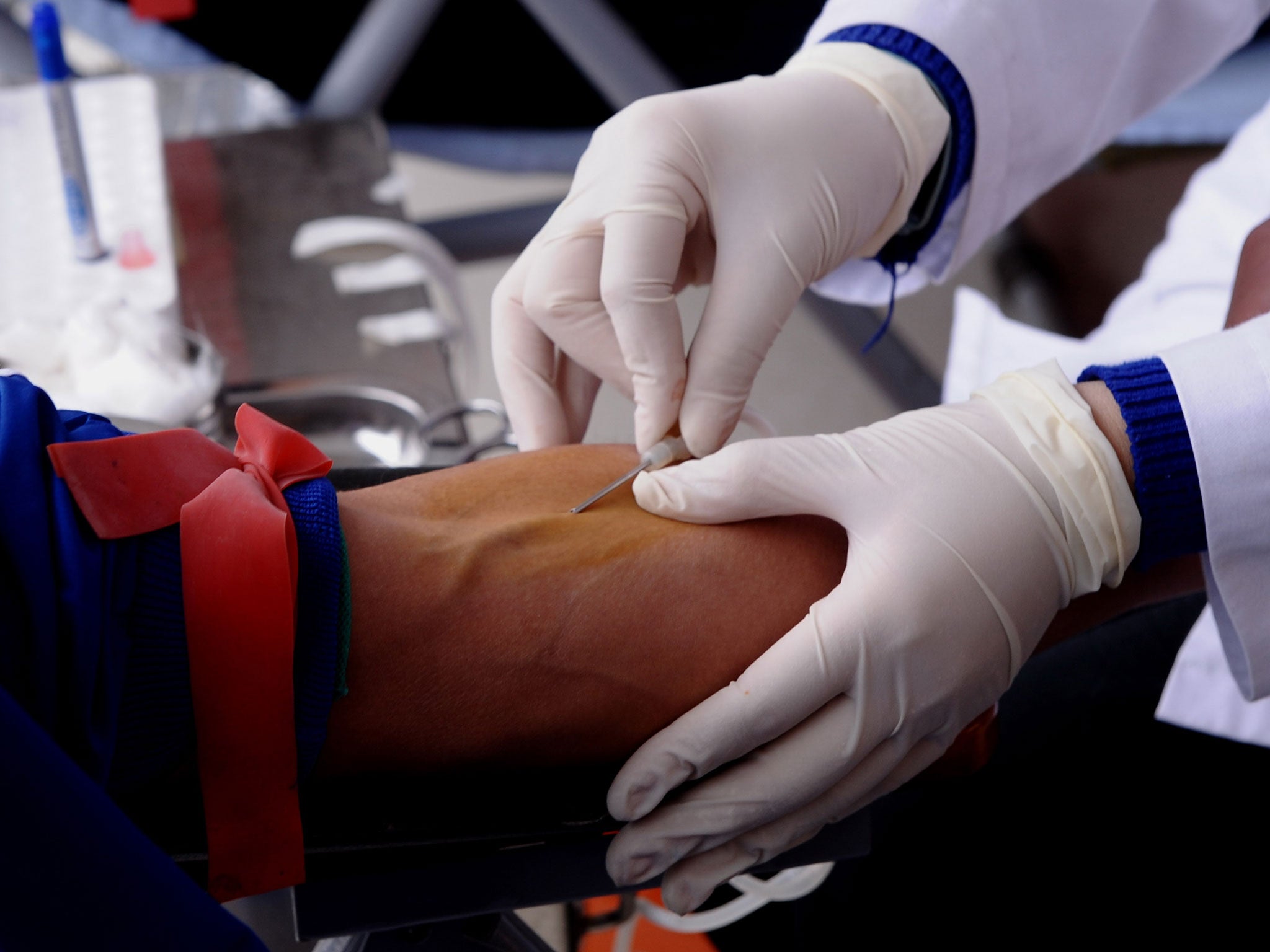
Your support makes all the difference.A new blood test for ovarian cancer has been found to detect twice as many cases as conventional methods, and is so successful it could lead to national screening programmes.
A groundbreaking medical trial carried out over 14 years in the UK - the largest of its kind in the world for ovarian cancer screening - correctly diagnosed 86 per cent of cases deadly and invasive epithelial ovarian cancer (iEOC).
Scientists said the "very encouraging" results showed the new test was able to offer effective screening for a disease that is traditionally very difficult to catch early. Its symptoms, including loss of appetite, abdominal pain and bloating, are common across a range of conditions.
The trial was led by University College London (UCL) and involved more than 200,000 post-menopausal women aged 50 and over who were randomly assigned different screening strategies.
And UCL's Professor Usha Menon said that the new test, involving tracking changing levels of the protein CA125 in the blood, could change the way doctors screen for ovarian cancer in the UK.
"There is currently no national screening programme for ovarian cancer, as research to date has been unable to provide enough evidence that any one method would improve early detection of tumours," Professor Menon said.
"These results are therefore very encouraging. They show that use of an early detection strategy based on an individual's CA125 profile significantly improved cancer detection compared to what we've seen in previous screening trials.
"The numbers of unnecessary operations and complications were within acceptable limits and we were able to safely and effectively deliver screening for over a decade across 13 NHS Trusts. While this is a significant achievement, we need to wait until later this year when the final analysis of the trial is completed to know whether the cancers detected through screening were caught early enough to save lives."
The trial's chief investigator Professor Ian Jacobs, president of the University of New South Wales in Australia, helped develop the CA125-tracking technique. He said the early results showed the method can be "an accurate and sensitive screening tool".
"My hope is that when the results of [the trial] are available this approach will prove capable of detecting ovarian cancer early enough to save lives," he said.
Cancer Research UK said the prospect of a blood test was "exciting", but added that more work was needed to confirm whether the new method would save lives. Further results from the ultrasound arm of the trial, and the impact of screening on cancer death rates, are expected later this year.
Additional reporting by agencies
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments