New research suggests 'female sperm' and 'male eggs' possible
Japanese researchers use skin cells from mice to create sperm and eggs that were later used to create a live birth
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.New research from Japan has suggested that it may be possible in the future for scientists to grow male and female reproductive cells from the opposite gender. In other words, to create sperm from women and eggs from men.
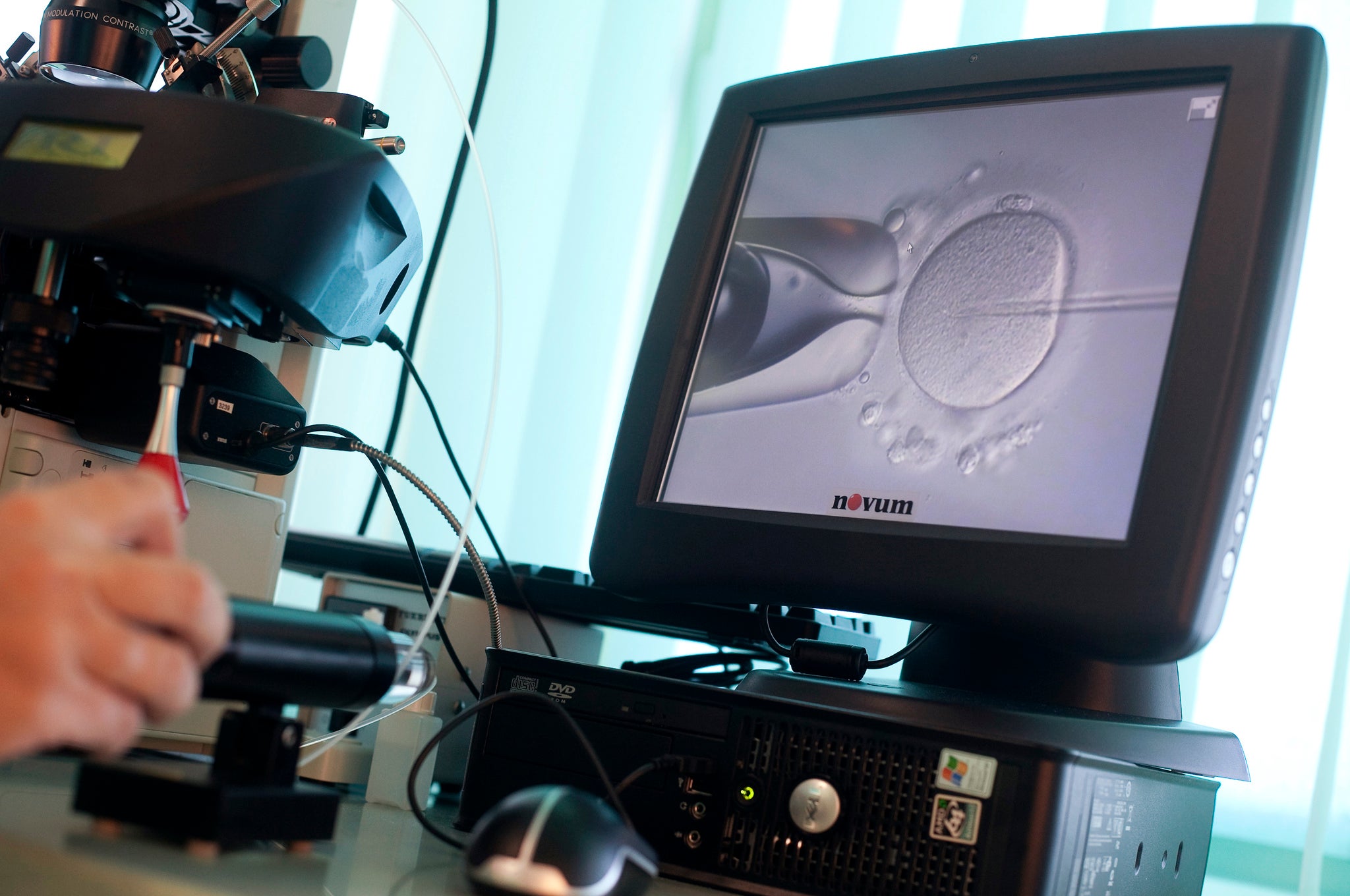
Katsuhiko Hayashi of Kyoto University in Japan has published research in which skin cells from mice were used to create primordial germ cells or PGCs. These cells, the common precursor of both male and female sex cells, were then developed into both sperm and eggs. Using these live-births were created via in vitro fertilisation.
Although the techniques involved are still in their infancy, the possibilities for reproductive medicine are startling. Not only could the research of Hayashi and his senior professor Mitinori Saitou allow infertile women to have babies by creating eggs from their skin cells, but it might make it possible for sperm and eggs cells to be created from either males or females.
The process begins by extracting pluripotent stem cells from early-stage embryos and somatic cells, and then converting these into PGCs using ‘signalling molecules’. These germ cells were transplanted into the ovaries and testes of living mice to develop. Once these cells were mature they were extracted and used to fertilise one another in vitro.
The initial research took place in October 2012, with the live-births only a ‘side effect’ used to demonstrate that the creation of PGCs had been successful. Since then, scientists around the world have been realising the full potential of the research, and the team involved is now exploring how their work might transfer to humans.x
Writing in Scientific American, David Cyranoski explains that other researchers have replicated the production of PGCs but have been unable to produce any live births. The scientists involved also have many other hurdles to overcome including the production ‘fragile’ and ‘misshapen’ eggs.
“But,” writes Cyranoski, “the most formidable challenge will be repeating the mouse PGC work in humans.” This is because the ‘signalling molecules’ used to create the PGCs are vastly more complicated in humans than in mice. Research is further hampered by restricted access to human embryos.
The Japanese team led by Saituou and Hayashi are currently using monkey embryos as a stepping stone between the species and when speaking to Scientific American, Hayashi predicts that they could succeed with primates within ‘5-10 years’, with the creation of human PGCs following ‘shortly after’.
However, even if the process is successfully transferred to monkeys there will still be many dangers that may take years to address. It’s already known that embryonic stem cells developed in laboratories frequently pick up various genetic mutations and epigenetic irregularities. Even if healthy offspring are created from this method, scientists have questioned how many generations would have to be observed before they are considered genetically ‘safe’.
Scientists agree that research is compelling but that it will be many years before anything like a viable treatment for infertility might be made available to the public.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments