IVF embryos to be genetically manipulated as scientists investigate repeated miscarriages
Critics say the 'Crispr' gene-editing technique could lead to 'designer babies'
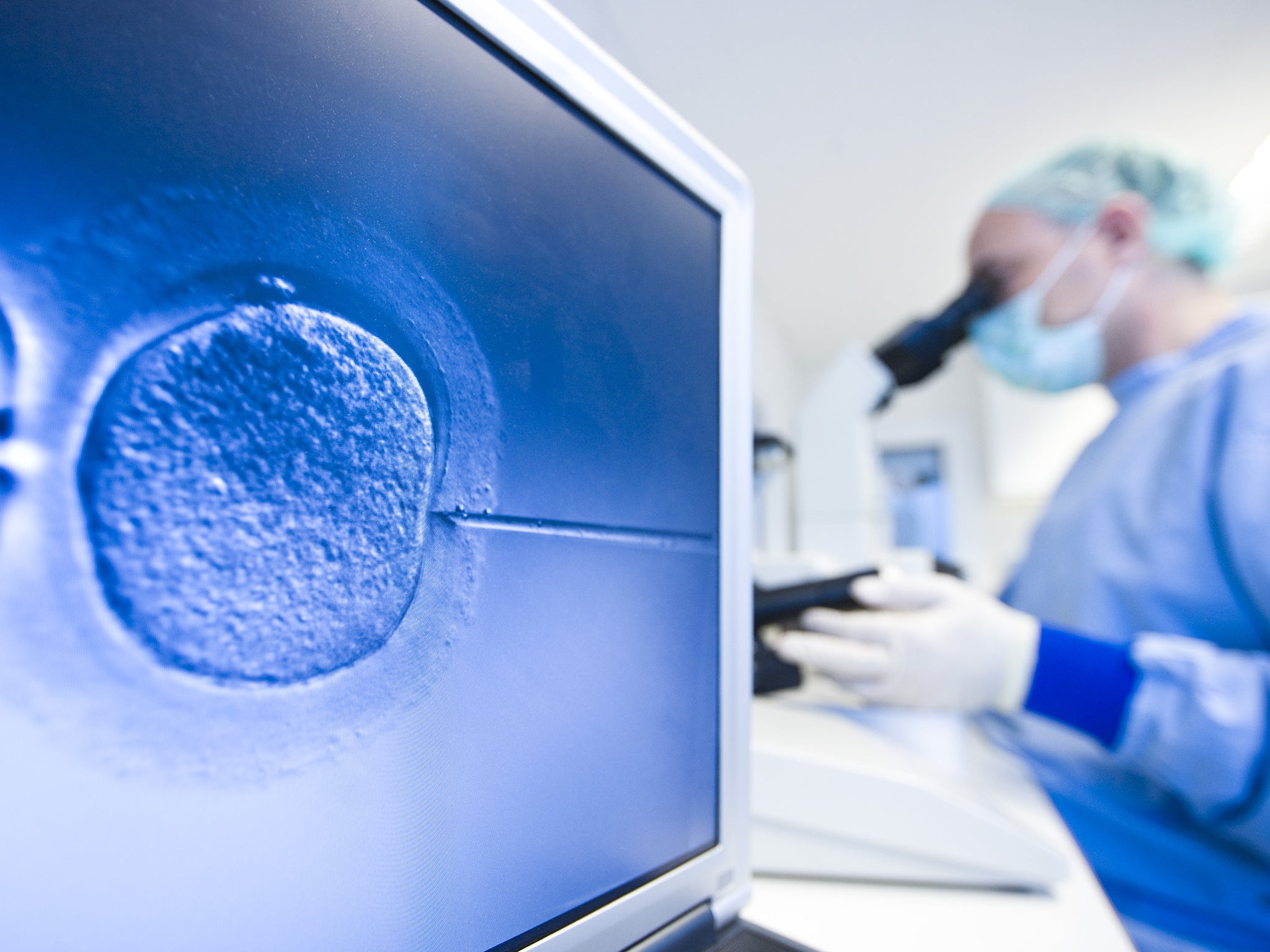
The genetic manipulation of human IVF embryos is set to start in Britain for the first time following a licence application by scientists who want to understand why some women suffer repeated miscarriages.
If the research licence is granted by the Government’s fertility watchdog it will be only the second known occasion in the world where the chromosomes of human embryos have been genetically manipulated using a revolutionary gene-editing technique called Crispr/Cas9.
When Chinese scientists announced earlier this year that they had genetically altered “spare” human IVF embryos using Crispr/Cas9 for research purposes, there was deep concern among many who thought that they had gone too far – the US Government later imposed a moratorium on federally-funded research in America.
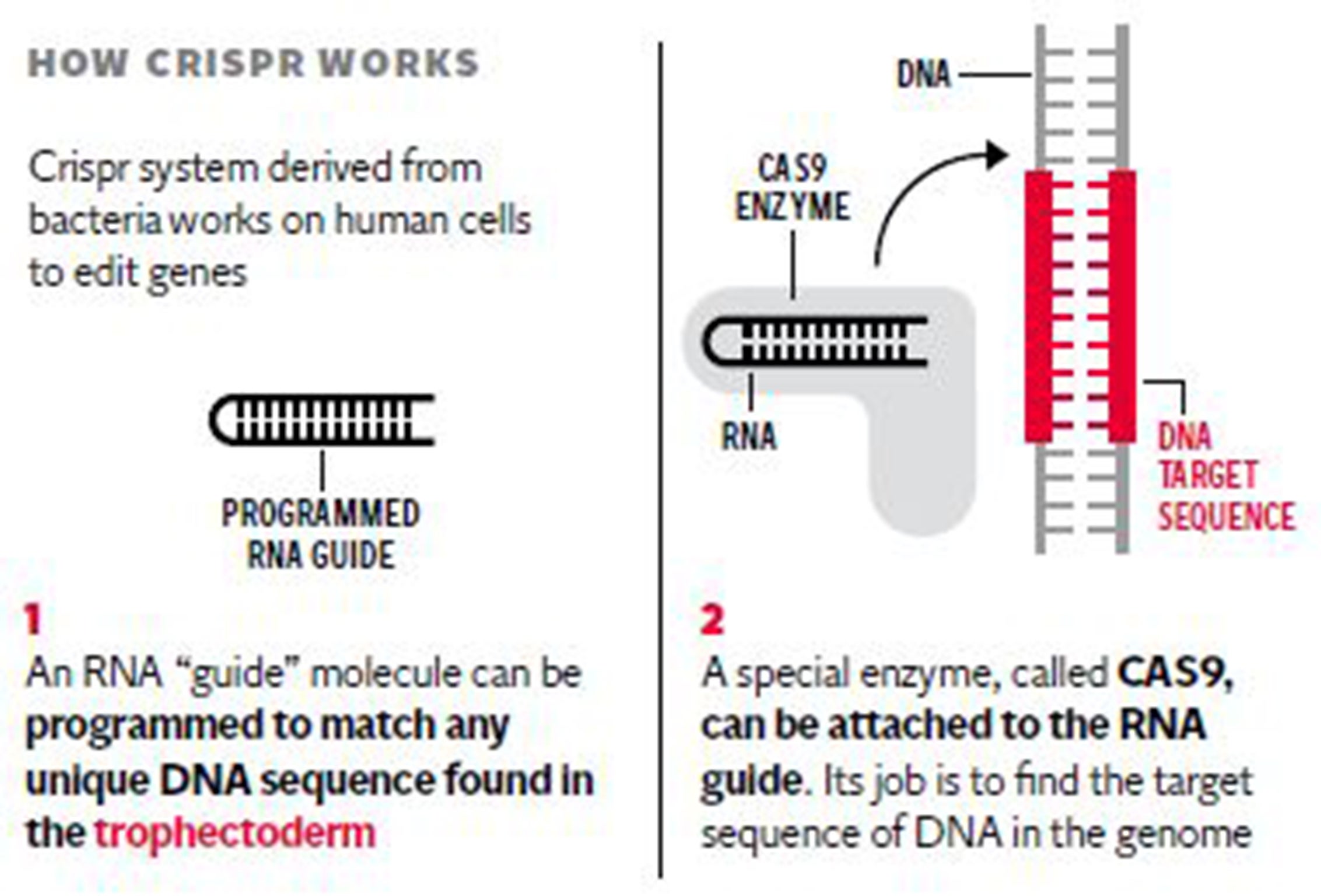
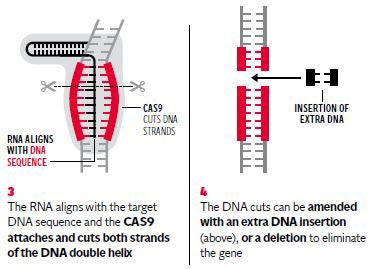
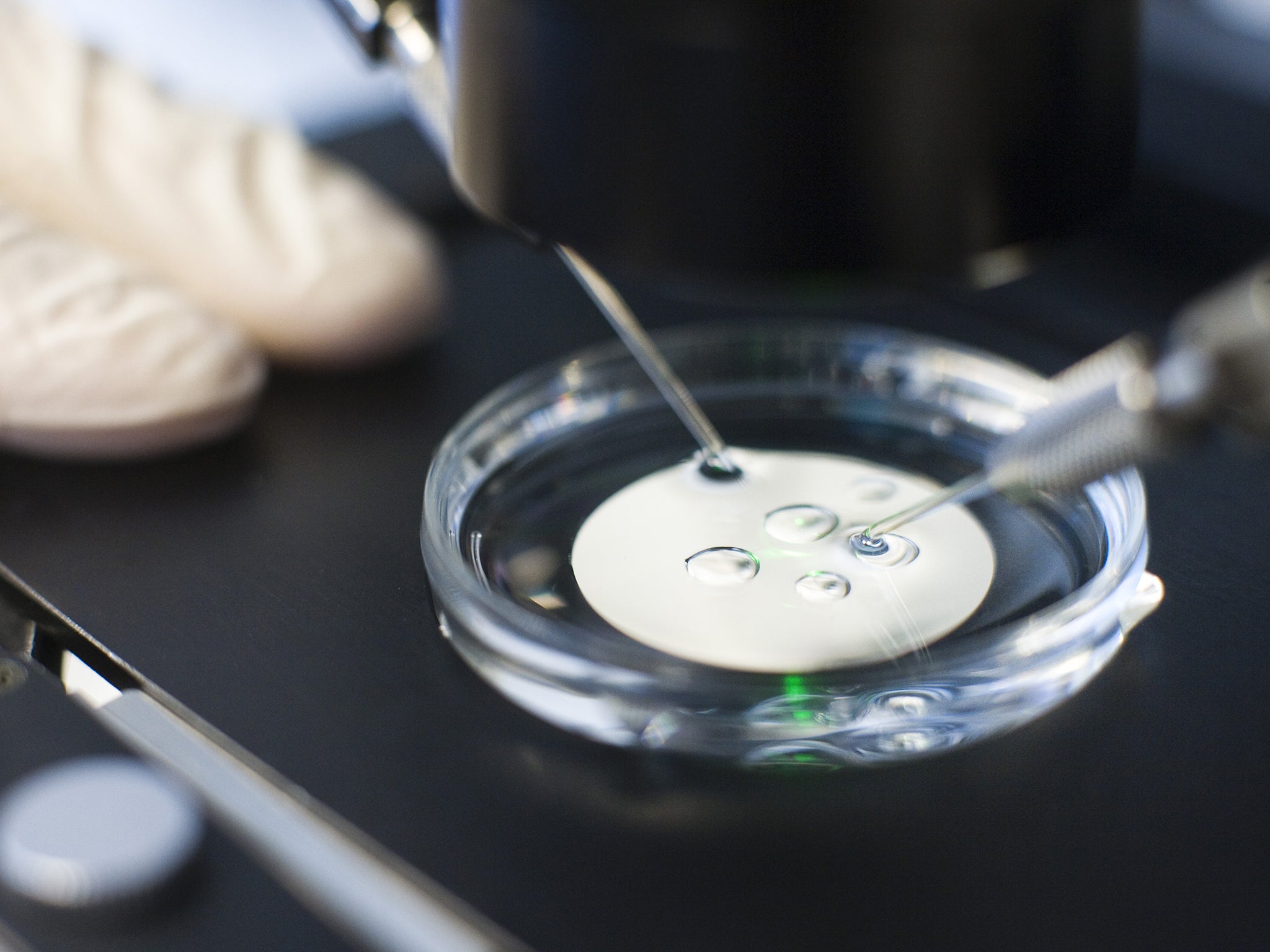
The researchers behind the UK application emphasised that the GM embryos will be destroyed once the study is completed, with no risk of them being transplanted into women – which is illegal in Britain. There will be no “GM babies” as the project is aimed solely at basic research into the genetics of early human development, they insisted.
However, critics of the manipulation of human IVF embryos – even when done for research purposes – have argued that it is a slippery slope to genetically-enhanced “designer babies” that might be engineered with desired traits such as intelligence or athletic ability.
The scientists behind the proposed study in the UK said they have no intention of altering the DNA of future generations but accept that this may at some point in the future be safe, medically justifiably and ethically acceptable – for instance to avoid inherited disorders or to confer disease resistance on IVF babies.
“We want to understand the genes human embryos need to develop successfully,” said Kathy Niakan of the Francis Crick Institute in London, who has applied to the Human Fertilisation and Embryology Authority (HFEA) for a research licence to use Crispr/Cas9 on spare IVF embryos donated by couples undergoing fertility treatment.
“We are not contemplating altering genes for clinical purposes – we are interested in basic mechanisms of embryonic development. If any of our discoveries suggest ways to improve embryo development after IVF, or to improve implantation frequency, or to prevent miscarriage, these would involve conventional approaches, not the manipulation of genes,” Dr Niakan said.
“There are suggestions that the methods could be used to correct genetic defects, to provide disease resistance, or even to introduce novel traits that are not found in humans. However, it is up to society to decide what is acceptable – science will merely inform what may be possible,” she added.
Parliament amended the UK’s IVF legislation in 2008 to allow genetic manipulation of embryos less than 14 days old, provided it was for research purposes and sanctioned by the HFEA. Under the HFE Act 2008, it remains illegal to create GM embryos for implanting into the womb, or to edit the “germline” DNA of chromosomes passed on to future generations.
“What we are proposing is in keeping with the HFE Act 2008, which is purely for research purposes. We hope to use this technology to improve our understanding of the earliest stages of human development,” Dr Niakan said.
“The knowledge we acquire will be very important for understanding how a healthy human embryo develops, and this will inform our understanding of the causes of miscarriage. The knowledge may also improve embryo development after IVF and might provide better clinical treatments for infertility,” she said.
“If we receive a licence, I would hope to start work as soon as possible. However, it is difficult to know how long it will take to carry out the project. In particular, we need to obtain sufficient embryos,” she added.
Professor Robin Lovell-Badge, a senior scientist at the Francis Crick Institute who took part in a major review of the ethical implications of Crispr/Cas9, a gene-editing technique that allows accurate and efficient engineering of the human genome, said that germ-line gene therapy is not on the agenda, and that Dr Niakan’s proposal is purely aimed at understanding why some women suffer repeated miscarriages.
“The ultimate clinical benefits could be improved methods of IVF and better implantation rates in women who have big problems maintaining a pregnancy because there is something wrong with the interaction between the placenta and the uterus,” Professor Lovell-Badge said.
“There is no intention at all [to do germline gene therapy]. It is purely a tool for the understanding the basic biology of early human development,” he said.

Both scientists emphatically denied that the licence application marks the start of a slippery slope to designer babies.
“Absolutely not. I don’t believe in the slippery slope anyway but within the UK we have very clear regulations. If you do any sort of manipulations on an embryo it is no longer a permitted embryo, it cannot be transferred into a woman. It is illegal to do so,” Professor Lovell-Badge said.
Dr Niakan, who moved from the US to the UK to carry out her research, added: “It is not a slippery slope because the UK has very tight regulation in this area, and it would be illegal to move in that direction….The HFEA has been instrumental in establishing a culture of proper discourse and regulatory oversight. At the moment, I believe the UK is the best place in the world to do this work.”
A spokesman for the HFEA said: “Genome editing of embryos for use in treatment is illegal. It has been permissible in research since 2009, as long as the research project meets the criteria in the legislation and it is done under an HFEA licence. We have recently received an application to use CRISPR-Cas9 in one of our licensed research projects, and it will be considered in due course.”
The Chinese experiment
The only known occasion when the gene-editing technique Crispr/Cas9 was used on human embryos was in China, in a study published last April. Researchers led by Junjiu Huang of Sun Yat-sen University in Guangzhou used Crispr on 86 “non-viable” IVF embryos left over from fertility treatment to modify the gene responsible for beta-thalassaemia, a potentially fatal blood disorder.
The researchers wanted to see whether it would be feasible to eradicate the disease by altering the diseased gene at an early point in embryonic development – although the non-viability of the embryos demonstrated there was never any intention of using them to produce GM babies. However, only a fraction of the embryos contained the replacement DNA – suggesting that the technique is still too immature for clinical use.
Profile: Kathy Niakan
Kathy Niakan has two first degrees from the University of Washington in Seattle: one in cell and molecular biology, the other in English literature. However, she chose to pursue science – in particular biology and genetics – when as an undergraduate she had her first experience of a top-level research laboratory.

After obtaining a PhD at the University of California, Los Angeles, she did postdoctoral research at Harvard University where she worked on mouse and human “pluripotent” stem cells, which can develop into the specialised tissues of the body.
She later moved to the UK to work at Cambridge University before being enticed to work at the National Institute for Medical Research at Mill Hill, which is now merged with the new Francis Crick Institute in London.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments
Bookmark popover
Removed from bookmarks