‘Mini organs’ grown from stem cells taken in late pregnancy
The research means human development can be observed in late pregnancy for the first time.
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.“Mini organs” have been grown for the first time using human stem cells taken during pregnancy, potentially leading to advances in prenatal medicine.
The study means human development can be observed in late pregnancy for the first time, raising the possibility of monitoring and treating congenital conditions before birth.
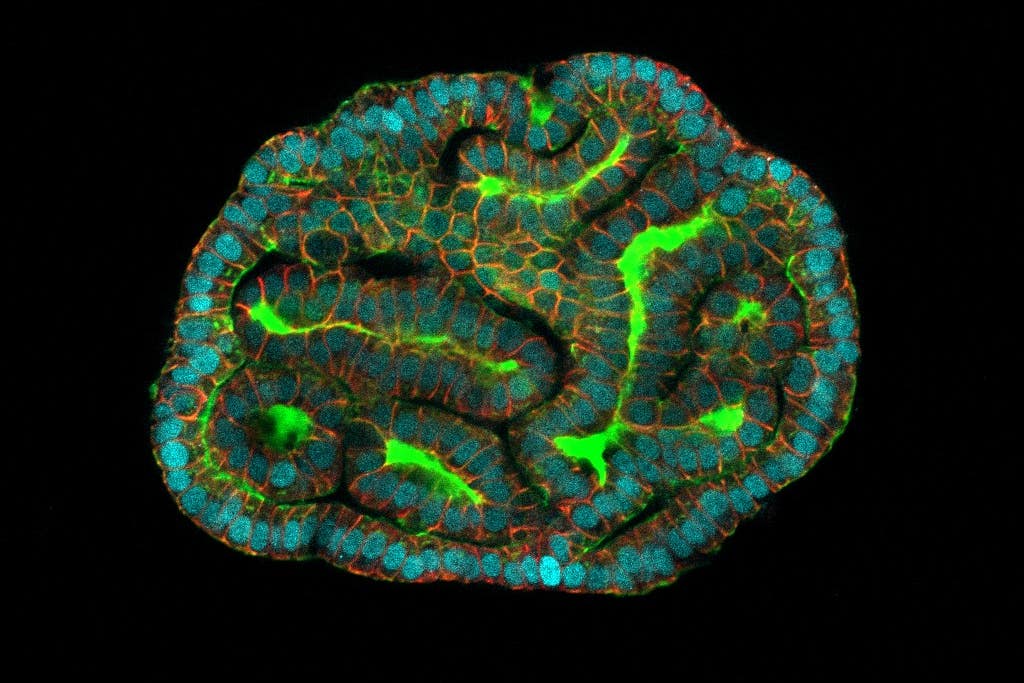
The research, published in Nature Medicine, sets out that complex cell models, called organoids, have been grown, and these “mini-organs” retain the baby’s biological information.
Organoids enable scientists to study how organs function both when they are healthy and when impacted by disease.
The researchers say the mini organs will facilitate monitoring of foetal development in late pregnancy, modelling of disease progression and testing of new treatments for diseases such as congenital diaphragmatic hernia (CDH) – a defect in an unborn baby’s diaphragm.
They (the organoids) will allow us to study what is happening during development in both health and disease, which is something that hadn’t been possible before
Dr Mattia Gerli, first author of the study at UCL Surgery and Interventional Science, said: “The organoids we created from amniotic fluid cells exhibit many of the functions of the tissues they represent, including gene and protein expression.
“They will allow us to study what is happening during development in both health and disease, which is something that hadn’t been possible before.
“We know so little about late human pregnancy, so it’s incredibly exciting to open up new areas of prenatal medicine.”
Until now organoids have been derived from adult stem cells or post-termination foetal tissue.
There are also regulations that restrict when foetal samples can be obtained.
In the UK this can be done up to 22 post-conception weeks, the legal limit for the termination of a pregnancy, but in countries like the US foetal sampling is illegal.
The regulations mean studying normal human development past 22 weeks has been limited, as well as for congenital diseases at a point when there may still be an opportunity to treat them.
To overcome these issues, researchers at UCL and Great Ormond Street Hospital (GOSH) extracted stem cells that had passed into the amniotic fluid, which surrounds the child in the womb and protects it during pregnancy.
Because the child is not touched during the collection process, sampling restrictions can be overcome and the cells carry the same biological information as the child.
A huge step forward for prenatal medicine
The researchers took live cells from 12 pregnancies – between the 16th week and the 34th week – as part of routine diagnostic testing.
They then identified which tissues the stem cells came from.
Stem cells from the lungs, kidneys and intestine were successfully extracted, and used to grow organoids that had functional features of these tissue types.
The team worked with researchers at KU Leuven in Belgium to study the development of babies with CDH, a condition where a hole in the diaphragm means organs like the intestine and liver get displaced into the chest, putting pressure on the lungs and hindering healthy growth.
Mini organs from babies with CDH both pre- and post-treatment were compared to organoids from healthy babies to study the biological characteristics of each group.
The study found significant developmental differences between healthy and pre-treatment CDH organoids.
However, the organoids in the post-treatment group were much closer to healthy ones, providing an estimate of the treatment’s effectiveness at a cellular level.
NIHR Professor Paolo de Coppi, senior author of the study from UCL Great Ormond Street Institute of Child Health and Great Ormond Street Hospital, said: “This is the first time that we’ve been able to make a functional assessment of a child’s congenital condition before birth, which is a huge step forward for prenatal medicine.
“Diagnosis is normally based on imaging such as ultrasound or MRI and genetic analyses.
“When we meet families with a prenatal diagnosis, we’re often unable to tell them much about the outcome because each case is different.
“We’re not claiming that we can do that just yet, but the ability to study functional prenatal organoids is the first step towards being able to offer a more detailed prognosis and, hopefully, provide more effective treatments in future.”
The researchers say that while they have not yet studied the method in relation to other conditions, it is possible they could look at other conditions that affect the lungs – like cystic fibrosis, kidneys and intestine.
Supported by the National Institute for Health and Care Research (NIHR) and Wellcome, the findings are published in Nature Medicine.