Inexpensive antidepressant can cut Covid hospitalisation risk, says study
There was up to 30 per cent hospitalisation reduction in fluvoxamine group
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
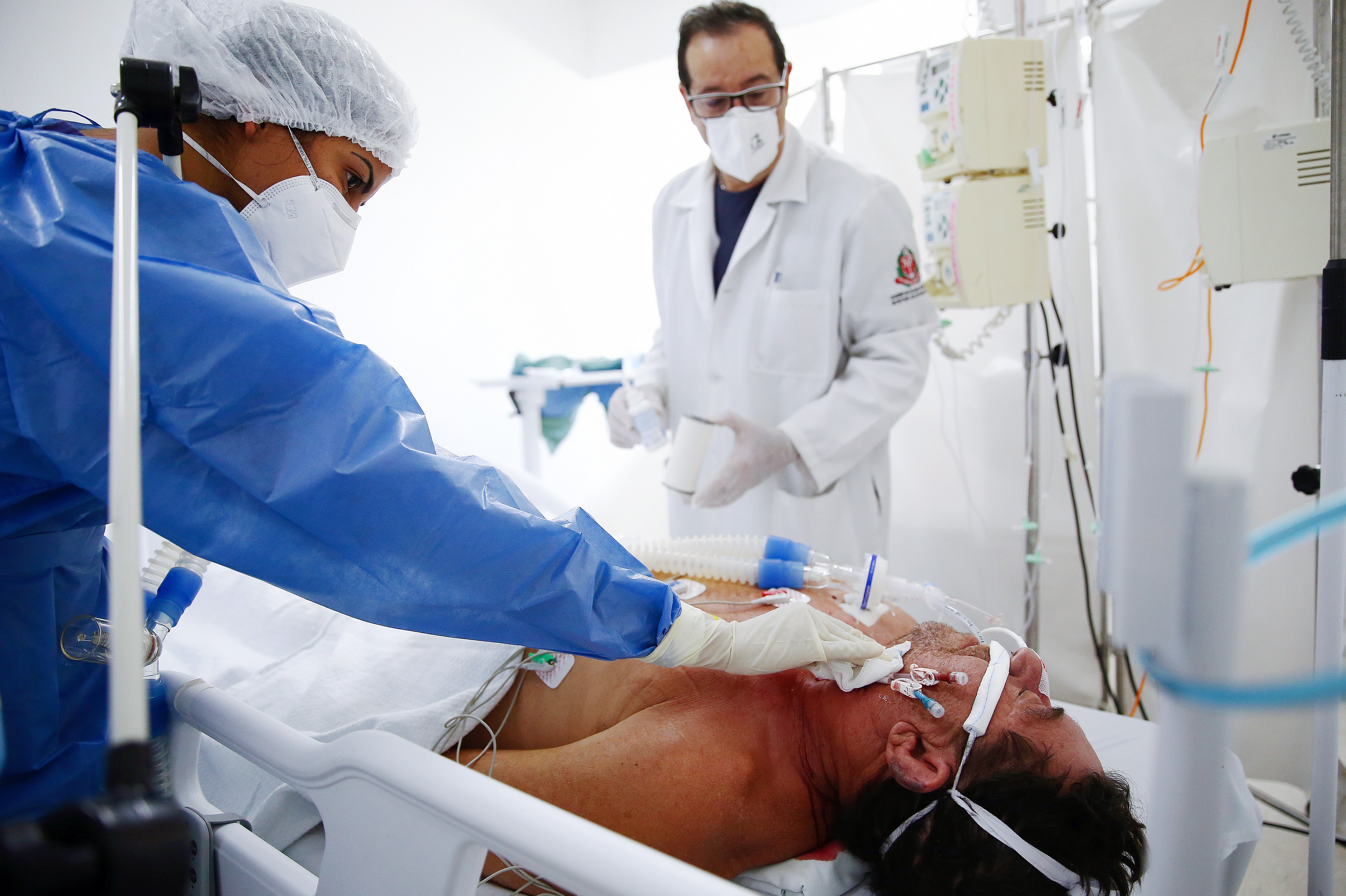
Your support makes all the difference.Early treatment of Covid-19 patients with the commonly prescribed antidepressant fluvoxamine can cut hospital admissions by up to 30 per cent, and potentially save lives, a new study has suggested.
The inexpensive drug could provide low-cost protection against severe Covid infection or death in low-income countries that are yet to receive adequate doses of vaccines, said the researchers from Brazil, US, Canada and Australia.
In the largest clinical trial of the drug conducted between 15 January and 6 August, 739 randomly selected Brazilian Covid-19 patients were treated with fluvoxamine, while another 733 received a placebo.
The researchers tracked every patient who had received fluvoxamine for 28 days to determine their health and if they still needed hospital treatment. They found that there was up to 30 per cent reduction in hospitalisations among those who had received 100mg of fluvoxamine twice daily for ten days, compared to those who had received the placebo.
The drug, commonly used to treat mental health conditions such as depression and obsessive compulsive disorder, was chosen to study its Covid-19 treatment potential due to its anti-inflammatory properties, noted the research, published in The Lancet journal.
Fluvoxamine was offered to patients in one of the arms of the “Together Trial” — a randomised adaptive platform trial to investigate the efficacy of eight repurposed treatments for Covid-19 among high-risk adult outpatients.
Of the 741 patients who received fluvoxamine, the scientists found that 79 required an extended stay for more than six hours in an emergency setting or hospitalisation, compared to 119 out of the 756 participants who received a placebo.
Costing about $4 per 10-day course, fluvoxamine could be a game-changer for low-income countries that have low vaccination rates and lack access to more advanced Covid-19 therapies, scientists said.
Researchers say the drug may have the potential to reduce severe immune response known as the cytokine storm seen in Covid-19 patients that can cause lethal organ damage.
Though reducing deaths was not an intended focus area in the study, the scientists noted that among patients who took at least 80 per cent of medication doses, there was one death in the fluvoxamine group. In the placebo group, there were 12 deaths.
“Given fluvoxamine’s safety, tolerability, ease of use, low cost, and widespread availability, these findings may have an important influence on national and international guidelines on clinical management of Covid-19,” Gilmar Reis, co-principal investigator, based in Belo Horizonte, Brazil, said in a statement.
The researchers hope to assess next whether the closely-related drug fluoxetine, which is part of the WHO Essential Medicines List, can be used interchangeably for Covid-19, and if combining fluvoxamine with other drugs could provide better treatment outcomes.
“Fluvoxamine is, so far, the only treatment that if administered early, can prevent Covid-19 from becoming a life-threatening illness,” Edward Mills, co-principal investigator for the “Together Trial” from McMaster University in Canada, said.
“It could be one of our most powerful weapons against the virus and its effectiveness is one of the most important discoveries we have made since the pandemic began,” Dr Mills added.
The scientists however caution that the use of interventions like fluvoxamine to prevent Covid-19 disease progression and hospitalisation is “critically dependent” on reliably identifying patients at the highest risk of deterioration in the early stages of infection.
“The study strongly suggests that fluvoxamine constitutes an effective, safe, inexpensive, and relatively well-tolerated option for the management of ambulatory patients with Covid-19, which is particularly useful for (but not limited to) low-resource settings,” the researchers wrote.
Since the patient population recruited for the study were required to have at least one factor for more severe disease to start the fluvoxamine treatment, the impact on more severe outcomes remains uncertain.
In a linked commentary, Otavio Berwanger of the Academic Research Organisation of Hospital Israelita Albert Einstein in Sao Paulo, Brazil, said some questions related to the efficacy and safety of fluvoxamine for Covid-19 patients remained open even though the drug was commonly prescribed for mental illnesses like depression.
“It remains to be determined whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” he noted.
It is also unclear, according to Dr Berwanger, whether these findings extend to other outpatient populations with Covid-19 such as those without risk factors for disease progression, those who are fully vaccinated, and those infected with recent variants.
“Going forward, the inclusion of patients with vaccine breakthrough infection in trials of community-based interventions will be important, and trial protocols should be amended to enable inclusion of this important subgroup with appropriate pre-treatment stratification and sample size adjustment to enable meaningful conclusions to be reached,” Penny Ward, visiting professor in pharmaceutical medicine at King’s College London, said.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments