‘The dominant forms of life on Earth’: Inside the British archive that holds 6,000 of the deadliest bacterial strains
The NCTC, which turns 100 this year, is home to species that can infect, sicken, maim and kill us all. Its existence is vital for any future medical breakthrough, writes Jennifer Pinkowski

Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.In the winter of 1915, Private Ernest Cable arrived at the No 14 Stationary Hospital in Wimereux, France, in a bad way. The British army’s soldiers stationed on the Western Front in the First World War were being ravaged by a variety of microscopic enemies. For Cable, it was Shigella flexneri, the bacterium that causes dysentery.
A military bacteriologist named Lieutenant William Broughton-Alcock took a sample of S flexneri from Cable’s body after he died on 13 March, 1915. It was likely kept alive in agar, sealed under paraffin wax, and was eventually renamed NCTC 1 when it became the very first specimen added to Britain’s National Collection of Type Cultures, the oldest library of human bacterial pathogens in the world devoted to sharing strains with other scientists. The collection turned 100 this year.
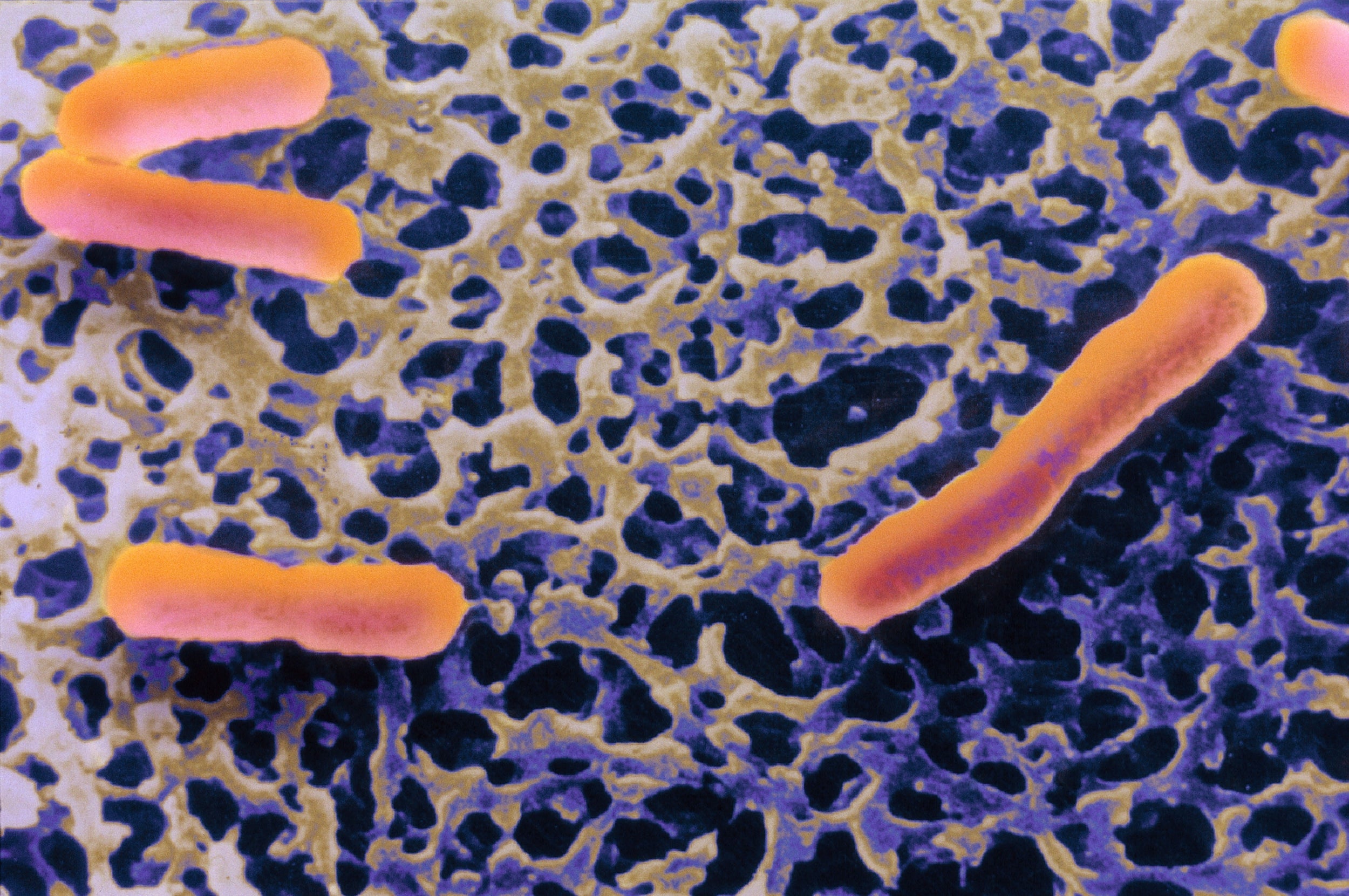
Managed by Public Health England, the NCTC holds about 6,000 bacterial strains representing more than 900 species that can infect, sicken, maim and kill us. (Strains are genetic variants of a species.) Of the nearly 800 registered culture collections in 78 countries, it is one of only a few dedicated to clinically relevant bacteria – that is, to species that make us sick.
About half of the microorganisms in the world’s culture collections are bacteria, dwarfing the number of viruses and fungi. While many scientists today are focused on fighting the spread of the novel coronavirus, bacteria continue to outmanoeuvre our immune systems and antibiotics. We think of them as invaders in our world, but really, we live in theirs.
“On any possible, reasonable or fair criterion,” writes Stephen Jay Gould, the evolutionary biologist, “bacteria are – and always have been – the dominant forms of life on Earth.”
The collection supplies many of the world’s clinical microbiologists with authenticated microbial strains of known origin. These scientists study how bacteria evolve; test safety protocols for infectious pathogens; develop vaccines, anticancer drugs and treatments for metabolic diseases; and study the ever-increasing problem of antimicrobial resistance.
Cable’s killer, for instance, was brought back to life from its freeze-dried form by Kate Baker, a microbiologist at the University of Liverpool, and her colleagues, part of an effort to understand how S flexneri has evolved over the last century. It still kills about 164,000 people every year, most of them children.
The team sequenced the NCTC 1’s genome and then compared it with other strains isolated in 1954, 1984 and 2002. Only 2 per cent of the bacterium’s genome had changed over the century, but those changes were associated with higher virulence, immune evasion and greater antimicrobial resistance.
When researchers like Baker discover a new species or strain, they can deposit it in the NCTC.
“Their science can then be reproducible, because other people can study it,” says Sarah Alexander, the collection’s lead scientist and curator. “There may be new applications for those strains.”
“From my perspective, it is one of the most important collections worldwide,” says Jorg Overmann, director of the German Collection of Microorganisms and Cell Cultures, one of the world’s largest and most diverse.
The collection first opened in London in 1920 at the Lister Institute of Preventive Medicine. Its first 200 cultures – including Cable’s – were deposited by Sir Frederick William Andrewes, a pathologist who studied dysentery throughout the First World War.
The organisation sent 2,000 strains to various institutions for free over the next year. The bacteria were delivered alive, teeming on a medium of agar made from Dorset egg yolks and sealed with paraffin wax.
It gives us this snapshot of an era for which we don’t have much information but that is critical for understanding how we’ve got to the antimicrobial crisis that we’re in today
Safety protocols were not in place yet: in 1922, three NCTC researchers caught Tularemia, or rabbit fever, during an experiment in which they had rubbed the spleen of a guinea pig infected with Francisella tularensis on the scarified skin of a healthy guinea pig.
The collection was transferred to a farmhouse north of London in 1939, a lucky move as the institute was bombed during the Second World War. In 1947, the curator honed the collection to medical and veterinary strains. The collection began charging scientists 2 shillings and sixpence per strain – about $5 (£4) today.
In the following decades, the growing collection moved back to London and raised its prices. Today it is a nonprofit that is self-supporting through the sale of strains, which usually cost between $85 and $375.
“I need to make sure the collections are scientifically relevant and financially robust,” says Julie Russell, the head of culture collections at Public Health England, which also has collections of pathogenic viruses and fungi.
The NCTC holds many bacteria relevant to medical breakthroughs. Alexander Fleming, who discovered penicillin, deposited 16 strains into the collection between 1928 and 1948. Fleming sourced NCTC 4842, the bacterium Haemophilus influenzae, from his own nose. Betty Hobbs, a noted expert on food poisoning who identified Clostridium perfringens as the culprit behind many food-borne illnesses, deposited more than 20 related strains.

A subset of its holdings is the Murray Collection, assembled by Everitt George Dunne Murray in the first half of the 20th century from the stool, urine, blood, cerebrospinal fluid and other bodily products of sick people across the world. The sub-collection’s 683 strains span the period when antibiotics entered into general use.
“It gives us this snapshot of an era for which we don’t have much information but that is critical for understanding how we’ve got to the antimicrobial crisis that we’re in today,” Baker says.
The collection has also sequenced the genomes of about half the strains, making that data available publicly for genetic research.
In 2019, the collection sent 3,803 ampuls of bacteria to 63 countries. Among the most requested genera and species were Clostridium (a leading cause of infectious diarrhea), E coli (360 strains, some dangerous, others harmless), Staphylococcus (causing infections ranging from mild to fatal), Mycobacteriaceae (responsible for tuberculosis and leprosy, among others) and Salmonella (from contaminated food).
They are shipped from a distribution centre outside London under strict protocols, with safe handling instructions. Most bacteria are biosafety level 2 or 3, which means they can cause serious or lethal diseases but have a cure. Level 4, the deadliest, includes only viruses.
The collection is also growing at a good clip.
“We receive anywhere between 50 and 200 strains a year from all sorts of sources,” says Jake Turnbull, a microbiologist at the collection.
Some are newly discovered, called type strains. Others are deposited from historical collections, or by scientists who retire or shift their research focus and want their strains to have a future.
Antimicrobial resistance is likely to be one of the most pressing public health concerns for years to come
New specimens are cultured on agar to make sure they are alive and uncontaminated, suspended in a sugar-rich cryoprotectant broth, freeze-dried at about -33C for three to four hours, plugged with steriliaed cotton, flame sealed in an evacuated glass ampul and stored at 4C. Not all specimens survive long-term storage.
“The process we use is very similar to the one developed in the 1930s,” Russell says.
Each sample must come with a description of its origin, identification and special characteristics that are added to a searchable database.
“Fifty years ago, you may just get a handwritten letter with a strain,” Alexander says. “Now we may get a strain that’s had its whole genome sequenced.”
They accept 90 per cent of samples they receive. Most are strains that are currently circulating, responsible for outbreaks or have novel antimicrobial resistance profiles.
One 2018 acquisition was NCTC 14208, a strain of Neisseria gonorrhoeae swabbed from the throat of a British man who recently contracted gonorrhea. The sexually transmitted disease, which infects nearly 80 million people every year, is becoming nearly untreatable.
“Evolutionarily, it’s quite an amazing bacterium,” Alexander says. “It has a lot of horizontal gene transfer. They swap antimicrobial resistance genes between the strains.”
The man had been given ceftriaxone and azithromycin – the only remaining treatment for the infection – but the bacteria beat them both. Another treatment eventually cured him.
In 2019, the collection sent 28 strains of N gonorrhoeae to dozens of researchers. The strain from the British man, referred to as “super gonorrhea”, went to six
In late February, the collection got a visit from Anna Dumitriu, who became its first artist-in-residence in 2018. Her work, often done in collaboration with scientists, frequently incorporates microorganisms.
Dumitriu extracted the super gonorrhea’s DNA in an NCTC molecular biology lab before getting a lesson from Alexander on proteomics, a tool for studying antibiotic resistance that involves analysing bacterial proteins.
“The loves of my life are chlamydia and gonorrhea,” Alexander, who has researched STDs for years, says during the session.
In anticipation of the collection’s 100th anniversary, Dumitriu created the raw-silk “Plague Dress”, impregnated with killed Yersinia pestis bacteria, which she extracted from the collection’s samples with Alexander’s help.
She also observed the process of how a strain becomes part of the collection, using a penicillin-resistant strain of Staphylococcus aureus swabbed from her own nose, a la Fleming, and added to the collection as NCTC 14139.
“I’ve used it in quite a lot of artworks and installations,” Dumitriu said.
She is not sure yet what creative expression the super gonorrhea strain will yield.
Antimicrobial resistance is likely to be one of the most pressing public health concerns for years to come. Of the 49 antibiotics currently in development, only four have been approved, and less than a quarter come from novel drug classes.
The samples being studied, donated and preserved in the NCTC and other culture collections will almost certainly play a role in medical breakthroughs decades in the future, just as Cable’s has a century after he died.
Alexander is keenly aware of this long-term view. Scientists who place their microorganisms in the collection “leave their legacy”, she says.
“You immortalise your science. We’re very much hopeful that in a hundred years’ time, people may be able to access strains that scientists deposited a hundred years ago.”
© The New York Times
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments