How Covid vaccines could help us in the fight against cancer
The same pioneering mRNA system that gave us Covid-19 vaccines could end up being key to helping cancer patients’ immune systems tackle the disease, writes Sean T Smith

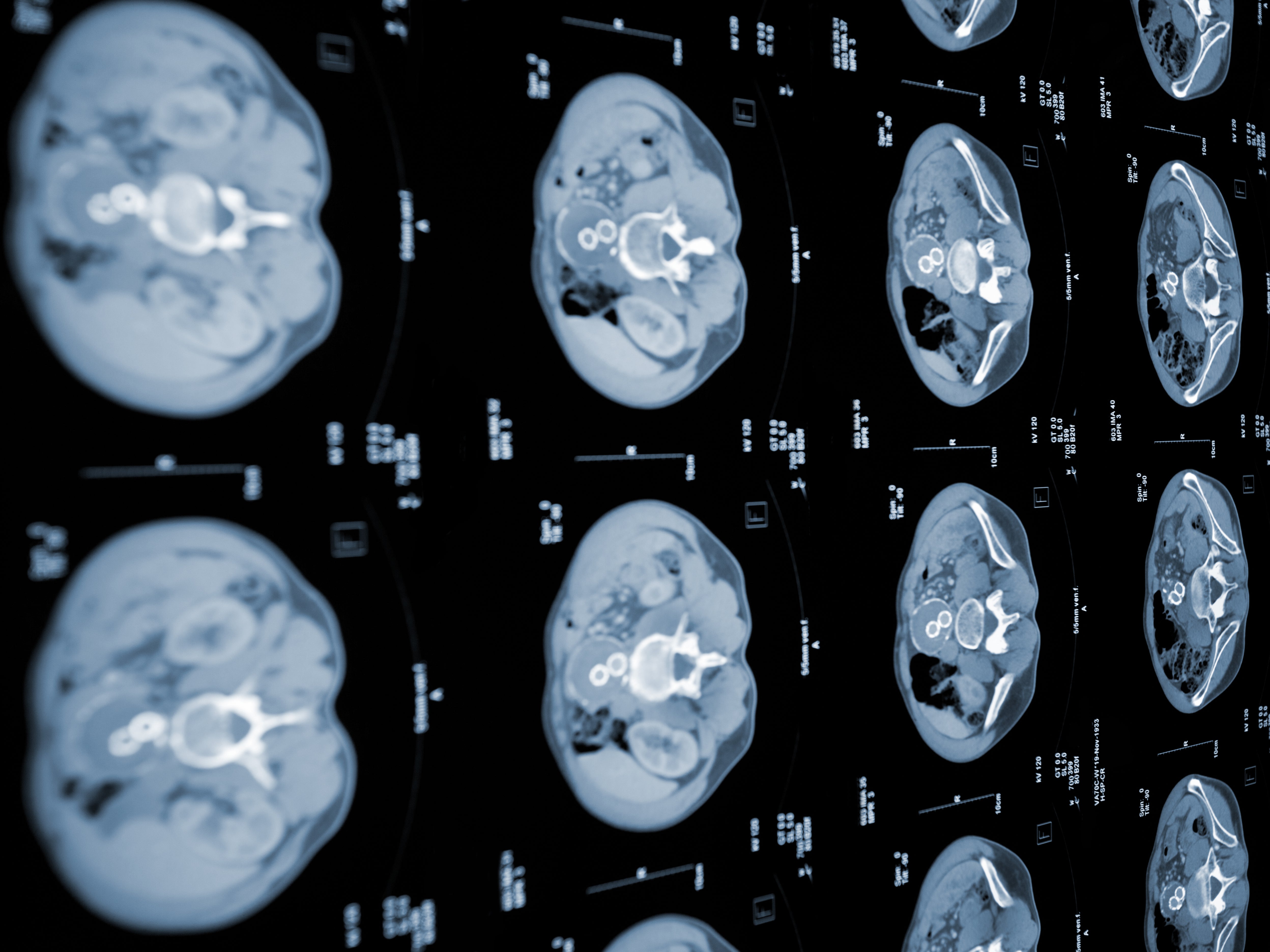
Patients are already benefiting from personalised cancer vaccines gleaned from the genetic damage found in their own biopsied tumours. Those vaccines are being delivered by the same pioneering mRNA system that gave us the Moderna and Pfizer-BioNTech Covid-19 jabs.
After decades of research and after finally being vindicated by its pandemic success, messenger RNA (mRNA) has established itself as a vaccine delivery platform so versatile it can be customised to carry individualised cancer vaccines.
Melanoma, for example, is the deadliest form of skin cancer because of the speed with which it can spread around the body, but its treatment is being revolutionised by mRNA vaccine technology that primes patients’ immune systems to fight back.
Since its Covid-19 success, Moderna has been trialling experimental melanoma vaccines. They’re made by identifying the mutating proteins (antigens) in a melanoma cell and then encoding genetic instructions into a molecule of messenger RNA. Once injected, the mRNA molecules train the immune system to mount an antibody response that targets the damaged proteins that are unique to that patient’s cancer.
In December, when Moderna announced that melanoma patients who had been “vaccinated” against their own cancers had seen a 44 per cent reduction in their risk of disease progression or death when the treatment was combined with an existing immunotherapy drug, it sparked enormous excitement in the cancer research community.
According to Dr Samuel Godfrey, research lead at Cancer Research UK, although the results are provisional, they offer the strongest hint yet that mRNA vaccine technology will be a major breakthrough in the fight against cancer . “This feels like the unlocking of the world of immunotherapy perhaps faster than would have been otherwise possible. It’s the first indication that we’re going to have another powerful tool to use against cancer and to complement chemotherapy and radiotherapy,” he tells me.
As professor of translational immunology at the Institute of Cancer Research in London, Alan Melcher agrees. He explains that although it has always shown enormous promise as a cancer treatment, immunotherapy has – up to this point – been something of a blunt instrument. “Sometimes even when it works we don’t always know why,” he explains.
He explains that when a tumour is establishing itself, its cells learn to shield themselves and hide from our immune system. That’s why the most commonly used immunotherapy drugs are checkpoint inhibitors that work by taking down this shield, allowing the immune system to get back to work attacking healthy and cancerous cells alike in an albeit generalised response that he compares to a “blunderbuss”.
Professor Melcher specialises in melanoma and head and neck cancers – the diseases that have been most responsive to the first generation of immunotherapy drugs.
For some patients, that generalised response is effective. But sometimes our immune systems need additional help to home in on the cancerous cells, and Dr Godfrey believes that’s where the new generation of mRNA cancer vaccines are likely to prove invaluable. “What’s beautiful about the mRNA technology is the speed at which you can personalise it to make the immune system recognise a tumour. It gives us a tool that we didn’t have before,” he says.
Strictly speaking, the new generations of mRNA cancer vaccines aren’t really going to be vaccines at all because they’re not actually designed to prevent disease. Instead, it would be more accurate to classify them as therapeutic cancer drugs because they’re likely to be used after surgery to prevent cancer from returning or spreading. By postponing recurrences, the treatment is likely to extend remissions and lifespans, sometimes indefinitely.
Flush from the success of the Covid-19 vaccine it co-developed with Pfizer, German giants BioNTech announced in January that starting this autumn they would begin intending to enrol NHS patients into an enormous medical trial that by 2030 would see 10,000 people receive personalised mRNA vaccines. “Our goal is to accelerate the development of immunotherapies and vaccines using technologies we have been researching for over 20 years. The collaboration will cover various cancer types and infectious diseases affecting, collectively, hundreds of millions of people worldwide”, says professor Sahin.
From BioNTech’s point of view, Dr Godfrey explains why the UK is the optimal setting to research mRNA technology. “The UK is the first country to genetically sequence every diagnosed cancer as a matter of routine, and it has potentially the best clinical infrastructure in the world because it has the largest single pool of patients”.
The invaluable data generated by 10,000 individual vaccine case studies will be more than just “big”.
“It’s huge! It’s colossal! If somebody had told me about proposed personalisation on that scale a couple of years ago, I probably wouldn’t have believed it,” says Dr Godfrey
Professor Melcher and Dr Godfrey expect Moderna and BioNTech to initially target the cancers that already respond well to existing immunotherapy treatments. Typically, that’s melanomas and head and neck cancers where there is very obvious damage to their DNA caused by factors like sunshine or smoking. That’s because our immune systems are much more likely to trigger a strong “immunogenic” response when it’s confronted by cells that look very unusual in a very obvious way.
“An antigen is an abnormal protein in a cell – a protein that shouldn’t be there”, Professor Melcher tells me. “Greater DNA damage means more abnormal proteins or antigens and therefore the greater the immunogenic response. That’s why smoking-induced lung cancer already responds to existing immunotherapy treatments much better than the [rarer] lung cancers that occur in non-smokers”, he explains.
At Cancer UK, Dr Godfrey shares the optimism that lung cancer – the UK’s third commonest form of the disease – is likely to be one of the significant beneficiaries of mRNA vaccine technology. Given that that there are 48,500 new lung cancer patients every year and that it accounts for 13 per cent of all cancers, this breakthrough is potentially very exciting.
Other particularly immunogenic forms of the disease that are expected to benefit from the first waves of concerted mRNA vaccine research include bladder, oesophageal and colorectal cancers, as well as some types of brain and breast cancer.
At the moment, immunotherapy doesn’t cope anywhere near as well with less immunogenic diseases like pancreatic cancer, for example where there is less obvious protein damage and rogue cells look genetically very similar to healthy cells. In these diseases, cancer-causing proteins are so much harder to spot; Dr Godfrey explains that if you were trying to use an mRNA vaccine on pancreatic cancer, for example, you probably wouldn’t see an immunogenic response anywhere near as significant as that demonstrated in Moderna’s melanoma trial.
But given anticipated advances in efficiency, speed and, crucially, the cost of genetic sequencing, it’s hoped that researchers will become better at flagging up subtler cellular anomalies, and so extending immunotherapy’s efficacy and therapeutic range. The pandemic has turbocharged investment in mRNA vaccine research. There’s even excitement that mRNA could be theoretically used to produce proteins missing in chronic conditions like sickle cell anaemia and cystic fibrosis.
The technology has brought the possibility of a universal flu vaccine into sharper focus, with clinical trials planned for 2023. Three mRNA HIV vaccines are also in clinical trials, and research is also under way to trial mRNA vaccines on Ebola and Zika. Although it’s hoped that mRNA vaccines will become a transformative technique that revolutionises cancer immunotherapy, question marks remain over its financial viability in both the short and long term.
It didn’t escape Dr Godfrey’s notice that the government’s announcement of the collaboration between BioNTech and the NHS occurred in January when the health service was on its knees. He admits to a degree of scepticism:
“No doubt part of it was to show us this shiny thing because it distracts from the fact that the NHS is also close to breaking point because of huge underinvestment. There’s little point investing in the science without investing in the infrastructure of doctors and nurses that allow clinical trials to thrive. If they don’t invest in that infrastructure it’s going to be difficult to realise those benefits,” he says.
And in the longer term, it’s by no means clear that a pathway from the first wave of personalised vaccines towards a mass-produced, affordable off-the-shelf cancer vaccine will ever materialise. For Professor Christopher Scott, dean of research at Queen’s University Belfast, this will be the critical stumbling block that will have to be overcome.
“At the moment we are focused on entirely personalised vaccines. This won’t work for most healthcare systems around the world – we need vaccines that can be mass-produced, on the shelf and ready to give a patient as soon as they are diagnosed with the disease and not made as a bespoke treatment for a single person with the cost and time implications incurred.
“If the technology proves as good as we hope then this will act as the stimulus to look at new versions that are either quicker to make or can be taken from the shelf. There does need to be movement here in order to generate a drug that offers more cost-effectiveness for healthcare systems around the world – not just the NHS,” he says.
But given that there’s no disease more genetically variable than cancer, Professor Melcher explains why a universal vaccine for any one specific form of the disease is unlikely.
“Some antigens are shared across tumours but most are personalised because they’re derived from the random DNA damage that happens to exist in an individual patient’s cell,” he says.
Dr Sam Godfrey agrees. For all of its promise, mRNA vaccine technology can never be a magic bullet. “But it’s almost certainly going to be the way we harness the immune system in conjunction with other treatments as part of a whole suite of tools.”





Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments