India tests vaccine delivery system with nationwide trial
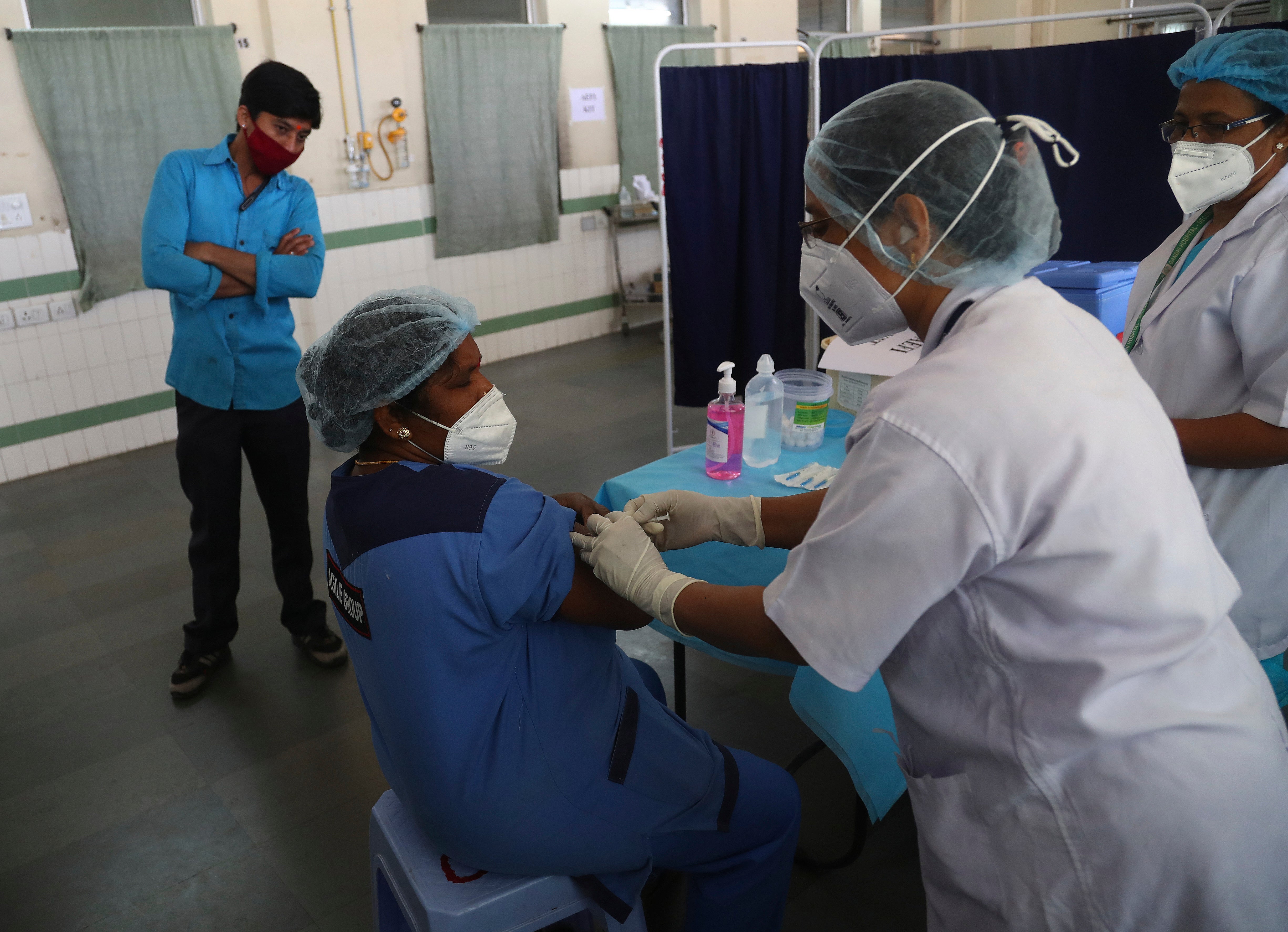
India has tested its COVID-19 vaccine delivery system with a nationwide trial as it prepares to roll out an inoculation program to stem the coronavirus pandemic
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.India tested its COVID-19 vaccine delivery system with a nationwide trial on Saturday as it prepares to roll out an inoculation program to stem the coronavirus pandemic
The trial included data entry into an online platform for monitoring vaccine delivery, along with testing of cold storage and transportation arrangements for the vaccine, the health ministry said in a statement.
The massive exercise came a day after a government-appointed panel of experts held a meeting to review the applications of potential vaccine candidates, including front-runner Covishield, developed by Oxford University and U.K.-based drugmaker AstraZeneca
India’s vaccination drive is expected to start in a few days once the country's regulator approves a vaccine.
The government plans to inoculate 300 million people in the first phase of the vaccination program which will include healthcare and front-line workers, police and military troops, and those with comorbidities who are above the age of 50.
The government is expected to initially lean on the vaccine produced by Serum Institute of India, the world’s largest vaccine manufacturing company. The company has been contracted by AstraZeneca to make 1 billion doses for developing nations, including India. It has applied to India's drug regulator for early approval for emergency use in the country. Applications for vaccines made by Pfizer Inc. and Indian manufacturer Bharat Biotech are also being reviewed.
Serum Institute of India's vaccine doesn’t require the ultra-cold storage facilities that some others do. Instead, it can be stored in refrigerators. This makes it a feasible candidate, not just for India but also for other developing nations.
Indian Health Minister Harsh Vardhan reviewed the preparedness for the vaccination drive at a government hospital in New Delhi on Saturday and urged the public not to pay heed to anti-vaccine rumors. “We will not compromise on any protocol before approving a vaccine,” he told reporters.
Pooja Moriya, a health worker in the capital who will be one of the first to be inoculated, said hospital staff has had several meetings about the vaccine and how it works. “Our seniors have told us to not be scared at all,” Moriya said.
India has confirmed over 10.3 million coronavirus cases, second in the world to the U.S. More than 149,000 people have died from the virus in India.