Government agrees to temporarily ban vaginal mesh implants for women with urinary incontinence
Campaigners hail decision as ‘vindication’ for women ‘maimed’ by operation
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
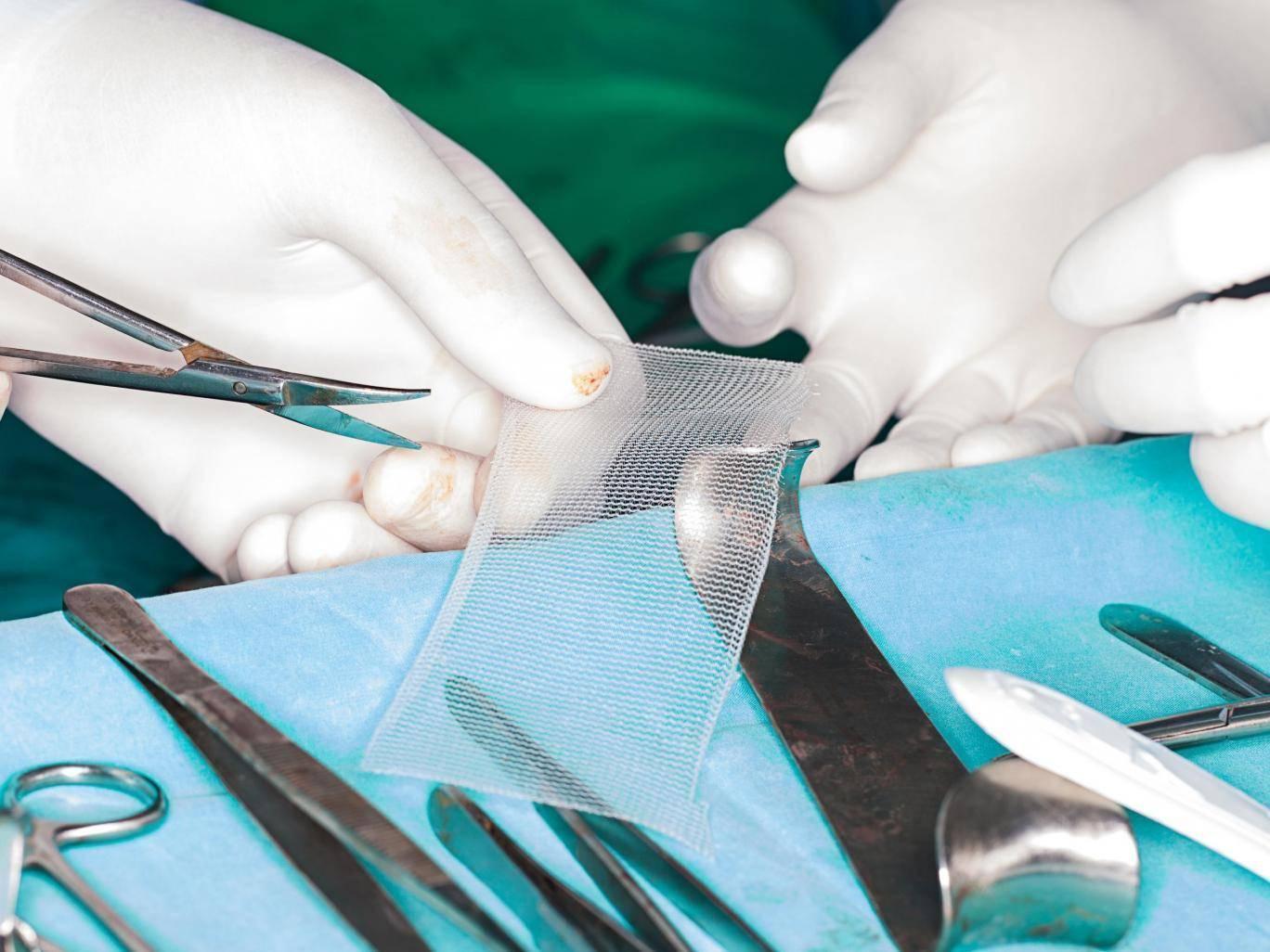
Your support makes all the difference.The government has accepted a recommendation to temporarily ban vaginal mesh implants for women with urinary incontinence.
The Independent Medicines and Medical Devices Safety Review concluded there must be an immediate pause in the use of surgical mesh to treat stress-urinary incontinence (SUI) – a condition where urine leaks out when the bladder is under pressure.
It comes after the National Institute for Health and Care Excellence (NICE) ruled last year against vaginal mesh as a treatment for pelvic organ collapse, but stated devices could still be used to treat SUI and to repair hernias in men or women.
Campaigners hailed the latest decision and said it was “vindication” for thousands of victims who were left in agony after undergoing vaginal mesh procedures.
Around 100,000 women are estimated by NHS England to have a mesh device fitted. Estimates vary over the extent of complications; NHS England figures say they occur in 3 to 5 per cent of women, but academics say it is closer to 10 per cent.
The risk rate of mesh to treat prolapse has been shown to be far higher than for incontinence or hernia: around 40 per cent.
Activists have predicted it could affect one woman in three, and politicians have called it the “biggest health scandal since thalidomide”.
The review looked into tens of thousands of cases where women were given harmful mesh implants. Baroness Julia Cumberlege, chair of the review, said the pause should last until March 2019, when conditions to mitigate the risk of injury could be put in place.
“We strongly believe that mesh must not be used to treat women with stress urinary incontinence until we can manage the risk of complications much more effectively,” she said in a statement. “We have not seen evidence on the benefits of mesh that outweighs the severity of human suffering caused by mesh complications.
“I have been appalled at the seriousness and scale of the tragic stories we have heard from women and their families. We have heard from many women who are suffering terribly.
“Their bravery and dignity in speaking out is deeply moving, and their sadness, anger, pain and frustration at what has happened to them and others has been compelling. We had to act now.”
The move is a win for campaigners, who are also pushing for a suspension of the use of mesh in rectopexy procedures. This will be considered separately by Baroness Cumberlege’s review.
"This is incredible news and vindication for more than 6,100 members of Sling The Mesh who have been maimed by this operation and then ignored, some for years,” said Kath Sansom, founder of campaign group Sling the Mesh.
"It is testament to people power. Our members have written, emailed, attended Parliament and lobbied to get this result and I am delighted.
"We now hope that Baroness Cumberlege adds rectopexy mesh to the suspension. This is used when patients suffer after a rectal prolapse. This is even more taboo and more embarrassing than urinary incontinence. Women suffer the same grave, life changing complications.”
Owen Smith MP, chair of the All Party Parliamentary Group on Surgical Mesh Devices, said: “This is wonderful news and it is long overdue, it is also a complete vindication of all those women who have campaigned tirelessly to suspend mesh and highlighted the damage the procedure has caused to many women.
“Baroness Cumberlege should be applauded for making this definitive statement just days after her review began taking evidence from women affected by mesh injury. She has obviously been left in no doubt – as those of us who have listened to women injured by mesh are in no doubt – that the risks far outweigh any benefits.
“Of course the reality of the situation is surgeons have already recognised that fact, which is why the use of mesh has declined so precipitously over recent years.”
He added: “I hope that ministers in Wales and Scotland and civil servants in Northern Ireland will follow suit and halt the procedure.”
Around 10,000 UK women a year are implanted with a plastic mesh device, which is inserted into the walls of the vagina to treat incontinence and prolapse – common side effects following childbirth.
The plastic is known to erode and disintegrate, and can slice through organs and vaginal walls.
Some women are unable to walk or stand properly, and almost all affected report chronic pain, infections and loss of sex life.
At least one woman has died following complications from her mesh implant. Chrissy Brajcic, 42, of Ontario, Canada, was left bedridden and in constant pain after the procedure, which was to treat minor incontinence.
Brajcic eventually became resistant to antibiotics used to treat her recurrent infections. She contracted sepsis and died of organ failure.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments