‘Life-changing’ sickle cell drug approved for NHS roll-out after patient-led campaign
Despite being the fastest-growing genetic condition in the UK, sickle cell disorder has historically been underserved by treatment options.
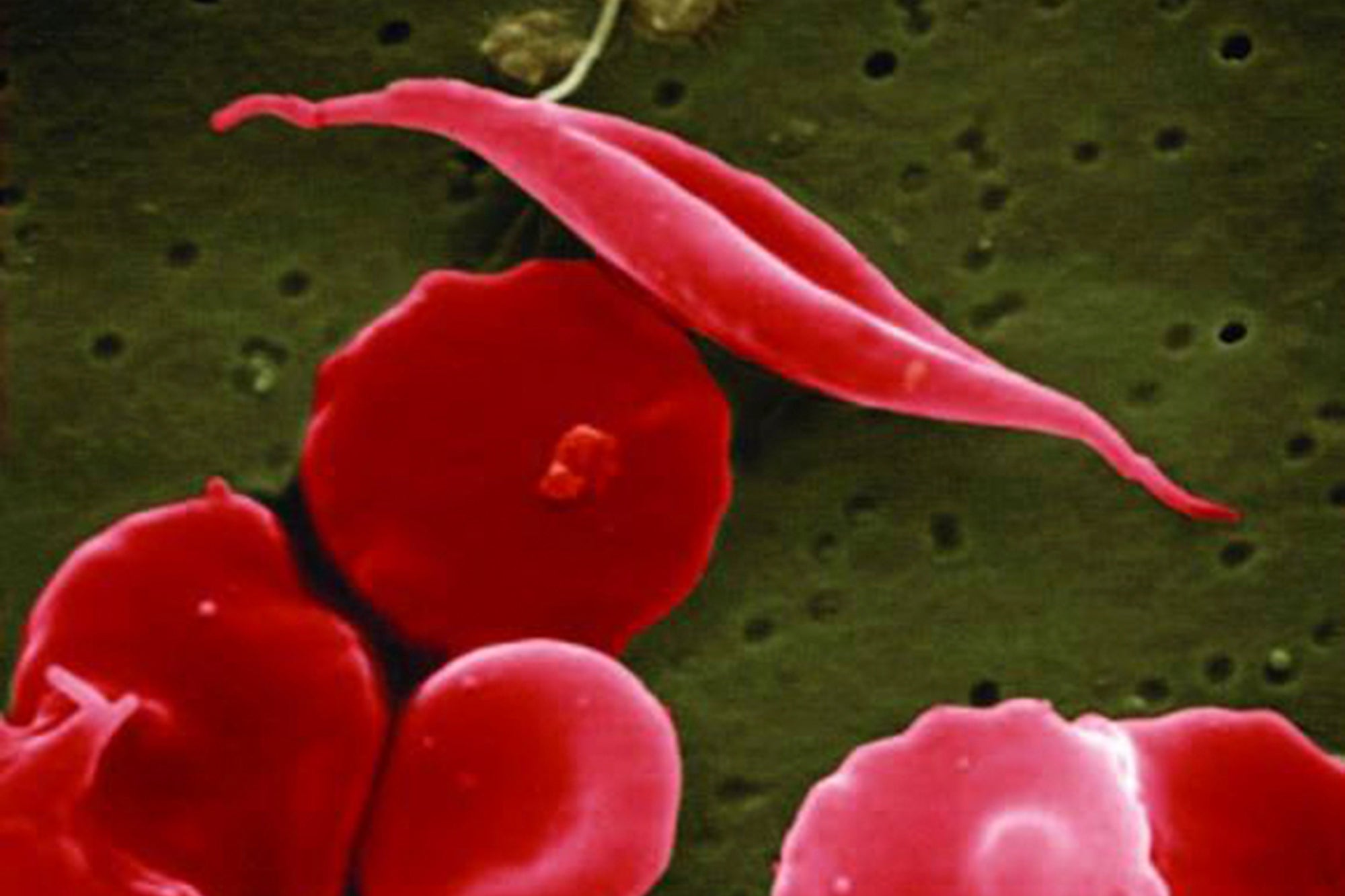
New treatment for sickle cell disease is set to be rolled out across the UK following a successful, patient-led appeal.
The drug, Voxelator, was initially rejected by UK health regulators last summer, which came as a blow to the 17,500 patients living with the disease in the UK.
This is the first sickle cell treatment to be recommended by the National Institute for Health and Care Excellence for routine use on the NHS.
“This decision from NICE has brought a great deal of hope, and we are profoundly grateful that this day has arrived. It is a deeply life changing and celebrated moment for people living with the condition,” John James, chief executive at the Sickle Cell Society said.
“Users of Voxelotor have shared with us remarkable stories of improved quality of life, reduced pain and substantial increases in energy levels. This has allowed them to work, connect with friends and family, improve their physical and mental health - and generally achieve a quality of life the rest of us often take for granted.
“We have seen decades of underinvestment in better care and safe effective treatments for sickle cell. For today we celebrate this milestone and commend everyone who tirelessly contributed to it.“
Mr James added: ”Tomorrow we’ll continue to advocate for a future where new treatments for sickle cell are not just a once in a lifetime occurrence for people living with the condition.”
Sickle cell disease predominantly affects Black people and this roll-out represents a landmark victory in the fight for fair access to safe and effective treatments.
Despite being the fastest-growing genetic condition in the UK, sickle cell disorder has historically been underserved by treatment options.
Crizanlizumab, hailed as first new drug to be introduced in over 20 years for people living with sickle cell disease, was axed in January following an internal review by regulators.
Only one other licensed treatment has been available to patients in the past two decades, while people who live with the illness have experienced racism, substandard care and negative attitudes from healthcare staff when accessing treatment.
Patients who use Voxelator have reported remarkable improvements in quality of life, including reduced pain and increased energy levels.
Sickle cell disease gets its name from the process of healthy, oxygenated, O-shaped red blood cells losing oxygen via an abnormal haemoglobin protein, causing the cells to turn C-shaped, resembling a farm tool called a sickle.
The cells then become hard and sticky, and get caught and clog up the blood vessels, which can cause severe pain known as crises, lead to frequent hospitalisation, delayed growth, strokes, lung problems and more.
Voxelator is taken orally and works to help increase red blood cell’s capacity to retain oxygen and prevent sickling.
Sickle cell patient Hazel Attua, who takes Voxelotor, hailed the NHS roll-out of this treatment as an opportunity for others “to experience the same transformation and embrace life to the fullest”.
“Being on Voxelotor for nearly two years has truly transformed my life,” she said. “Since starting Voxelotor, my energy has soared, making a massive change to my daily routine.
“This has not only improved my physical well-being but also my mental health, and I have a more positive outlook on life. I’m thrilled that others will have the opportunity to experience the same transformation and embrace life to the fullest.”
Susan Rienow, Country President at Pfizer UK which helped to develop the treatment, said: “We are delighted with this positive NICE recommendation and interim funding through the Innovative Medicines Fund, which will give eligible patients in England immediate access.”
This comes after a world-first gene therapy for severe sickle cell disease called Casgevy was recently rejected for widespread NHS use which prompted criticism from campaigners and patients.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments
Bookmark popover
Removed from bookmarks