The Independent's journalism is supported by our readers. When you purchase through links on our site, we may earn commission.
Nasal spray flu vaccine approved for home use: Here’s what to know
Vaccine provides an alternative to flu shots administered with needles
The Food and Drug Administration (FDA) has approved the first nasal spray flu vaccine which can be administered by the patient or a caregiver.
FluMist, produced by European pharmaceutical company and Covid vaccine-maker AstraZeneca, was announced last week and can be used by adults aged 49 and under.
With the assistance of a parent or caregiver, children and teenagers between the ages of two and 17 can also take the vaccine.
FluMist was initially approved by the agency in 2003 for individuals between the ages of five and 49, and has been used safely for years by medical professionals. In 2007, the FDA expanded its use to include children between the ages of two and five years old.
Hundreds of millions of doses have been distributed around the world since the spray was first approved.
FluMist will still require a prescription and will be available from an online pharmacy next fall.
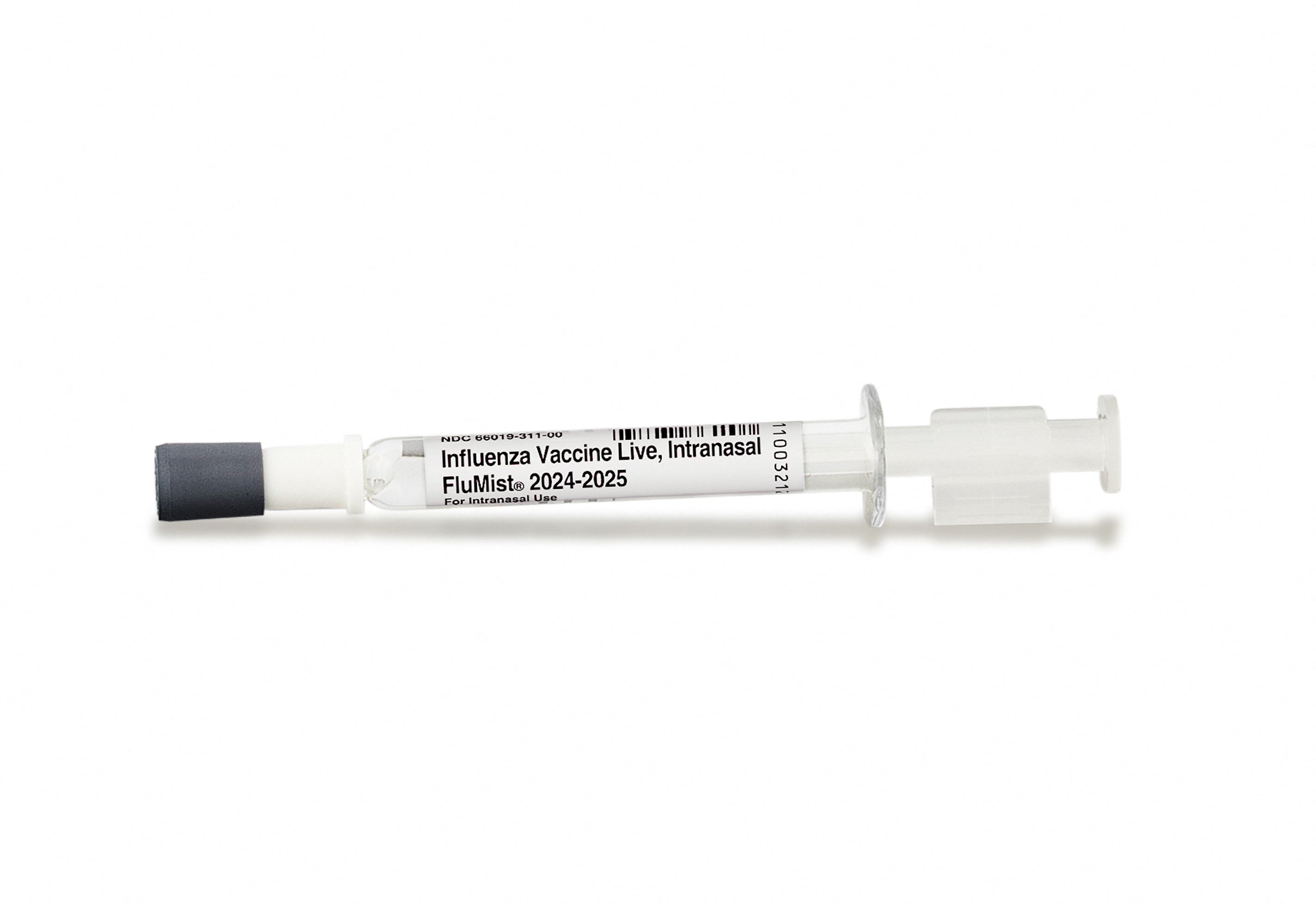
While patients can still receive FluMist in offices and pharmacies, AstraZeneca said it would launch a website where users can fill out a questionnaire to get the vaccine. Those answers will be reviewed by a pharmacist before the treatment is shipped out.
The current out-of-pocket cost for a dose ranges between $35 and $45, but it may cost less depending a patient’s insurance coverage, according to The New York Times.
Dr Peter Marks, the director of the FDA’s Center for Biologics Evaluation and Research, said on Friday that the approval provides more convenience, flexibility, and accessibility for individuals and families who want to be vaccinated for seasonal influenza.
“Getting vaccinated each year is the best way to prevent influenza, which causes illness in a substantial proportion of the U.S. population every year and may result in serious complications, including hospitalization and death,” he said.
Although seasonal flu viruses are detected year-round in the US, they circulate more easily during fall and winter months.
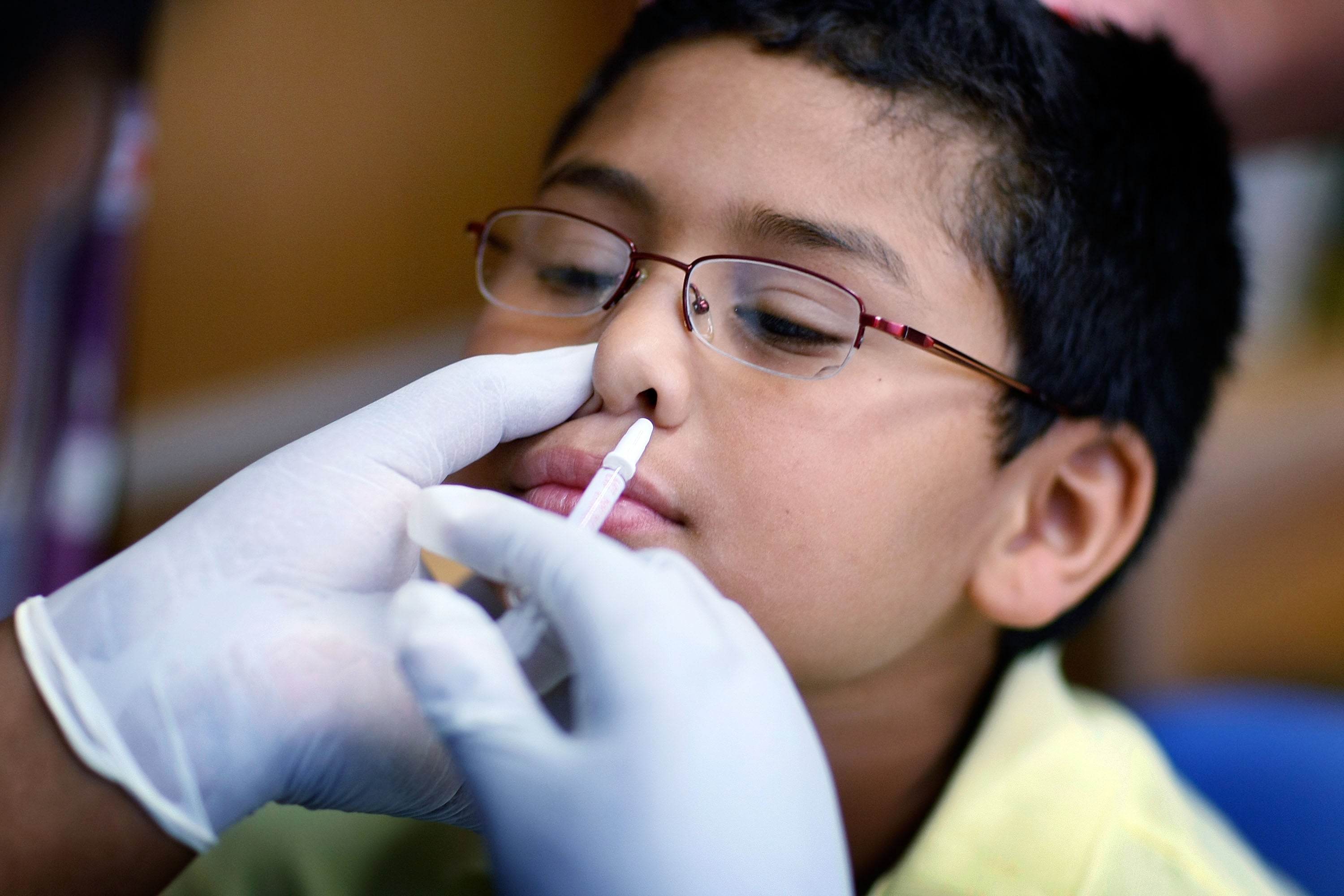
People are more susceptible to contagious respiratory illness as they head indoors and their immunity does not function as well in cooler temperatures and lower humidity.
Flu can result in mild to severe illness, and in some circumstances, death. It is unknown exactly how many people die from seasonal flu each year, but the Centers for Disease Control and Prevention (CDC) reported nearly 6,000 deaths in 2022.
Flu activity typically increases in October, with peaks between December and February. Children and adults who are 65 and older are the most susceptible to infection.
The CDC recommends that everyone six months and older receive an annual flu vaccine. Most of them are flu shots, given to patients through an injection in their arm. The vaccines protect against the four strains of influenza that researchers find will be most common during the season.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments
Bookmark popover
Removed from bookmarks