World-first cannabis-based medicine trial on babies begins in UK hospital
Treatment could be used to prevent seizures and brain injuries in newborns
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.The world’s first cannabis-based medicine trial on babies has begun in the UK, with researchers saying it is the first step in such a treatment being used to help newborns at risk of seizures and brain injury.
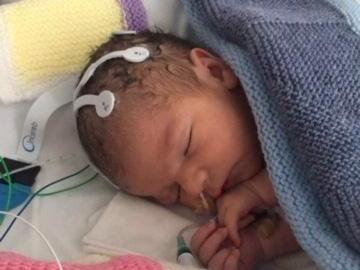
Within hours of his birth in an emergency caesarean, Oscar Parodi became the first baby in the world to join the drug trial at the Neonatal Intensive Care Unit (Nicu) at the Norfolk and Norwich University Hospital.
Researchers are investigating whether the cannabis-derived medicine could lessen the degree of brain injury for babies with neonatal hypoxic-ischemic encephalopathy (HIE), which is caused by a lack of oxygen or blood flow to the baby from the placenta.
Professor Paul Clarke, consultant neonatologist at the hospital, said there was “a lot of excitement on the unit” about the study.
“This is the first time a cannabis-derived medicine has been tested intravenously in human babies,” he said. “It is hoped that it will be good for preventing seizures and protecting the brains of newborn babies with HIE.”
Two babies have been enrolled in the randomised trial so far.
They will continue to receive standard hypothermia treatment for HIE, where their body is cooled to 33.5C, while receiving a single dose of the drug or a placebo.
The researchers will then conduct tests to measure levels of the drug in the blood.
Oscar, who was delivered by emergency caesarean section on 11 March, was unexpectedly born in a poor condition and transferred to the Nicu, where he was placed on the cooling therapy for 72 hours.
His mother, Chelsea Parodi, said: “I was approached after the birth about taking part in this study and I consulted my mum and my brother who is training to be a paramedic.
“It was hard but I wanted to do everything I could to help my baby boy.
“Oscar was in hospital for nine days and he was being monitored 24/7. He is doing fantastically well and I am really grateful to Dr Clarke and the team for what they have done for us.”
Prof Clarke said: “We have always had good support from families wanting to take part in research on our Nicu and they often do it from an altruistic point of view to help benefit future babies.
“One of the attractions of this trial for parents is the closer brain monitoring that babies get as part of the study, because a more advanced brain wave monitor is used for the trial babies.
“This gives parents more reassurance that any seizures will be picked up.”
He added: “As with any study of a new medicine, there may be unexpected side effects and unknown risks.
“With this in mind, the trial has been carefully designed to make it as safe as possible and so we are only giving the babies a minuscule dose at the beginning and we monitor them even more closely than usual.”
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments