Breakthrough new NHS drug can delay spread of breast cancer
Drug gives patients an extra 22 months of life on average
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
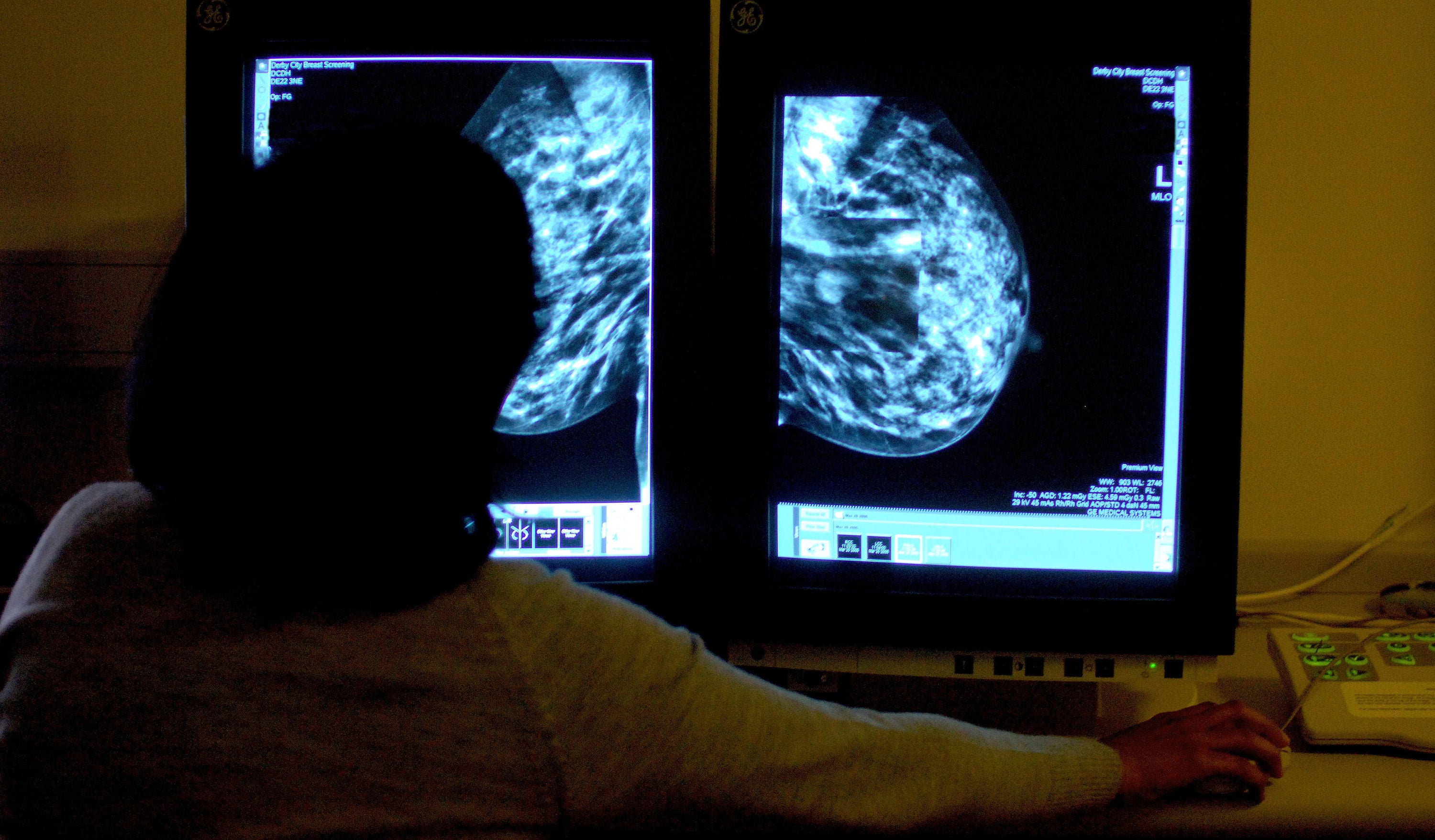
Your support makes all the difference.Breast cancer sufferers will be granted extra months of life thanks to a new drug that has been approved for NHS use.
Enhertu, or trastuzumab deruxtecan, will be rolled out to 600 women a year in England. Trials have shown that that drug improves the survival of recipients by halting the spread of tumours.
It gives patients an extra 22 months of life, on average, before the cancer progresses.
The breakthrough drug was approved for use on Monday by the National Institute for Health and Care Excellence (Nice). The treatment will be offered to breast cancer patients who have a type of the disease that tests positive for a protein called human epidermal growth factor receptor 2 (HER2).
Around a fifth of breast cancer patients test positive for this protein. The drug Enhertu finds and blocks the HER2 protein on cancer cells, while also unloading a powerful cancer-killing chemical inside those cells.
It is administered by intravenous drip once every few weeks and patients will be eligible if their cancer has not responded to other treatments.
Baroness Morgan of Drefelin, chief executive of Breast Cancer Now, told The Times that the drug’s approval was “fantastic news”.
She added: “This targeted treatment can significantly slow the spread of the disease compared to the current standard treatment, giving people more time to continue doing the things that matter to them.
“This decision highlights the continued importance of the Cancer Drugs Fund in enabling promising treatments to reach patients on the NHS quickly.”

Professor Peter Clark, head of NHS England’s Cancer Drugs Fund, which arranges access to promising new treatments, said: “This cutting-edge drug will give hundreds of patients with secondary incurable breast cancer hope.
“The NHS is committed to providing the very best treatments for its patients and trastuzumab deruxtecan is just the latest of more than 100 cancer treatments that have been fast-tracked for us on the NHS through the Cancer Drugs Fund, benefiting more than 80,000 patients.”



Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments