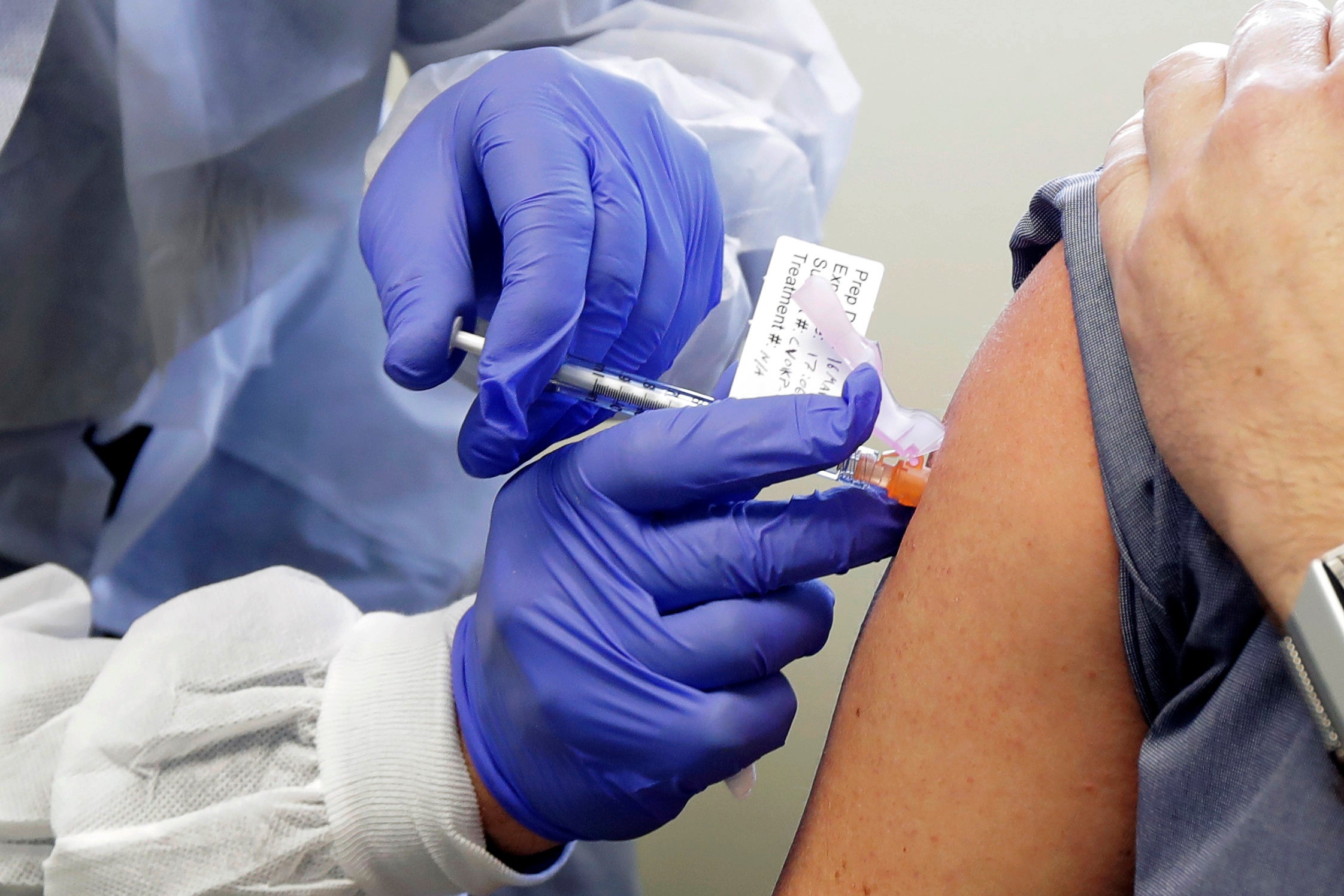
CDC panel meets Tuesday to vote on COVID-19 vaccine priority
A panel of U.S. advisers will meet this week to recommend who should be first in line for a COVID-19 vaccine
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.A panel of U.S. advisers will meet Tuesday to vote on how scarce, initial supplies of a COVID-19 vaccine will be given out once one has been approved.
Experts have proposed giving the vaccine to health workers first. High priority also may be given to workers in essential industries, people with certain medical conditions and people age 65 and older.
Tuesday's meeting is for the Advisory Committee on Immunization Practices, a group established by the Centers for Disease Control and Prevention. The panel of experts recommends who to vaccinate and when -- advice that the government almost always follows.
Pfizer and its German partner BioNTech have asked the Food and Drug Administration to allow emergency use of its COVID-19 vaccine candidate. Moderna Inc. is expected to also seek emergency use of its vaccine soon.
FDA's scientific advisers are holding a public meeting Dec. 10 to review Pfizer's request, and send a recommendation to the FDA.
Manufacturers already have begun stockpiling coronavirus vaccine doses in anticipation of eventual approval, but the first shots will be in short supply and rationed.