Canada approves Pfizer's COVID-19 vaccine for kids
Canada’s health regulator has approved Pfizer’s kid-size COVID-19 shot
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
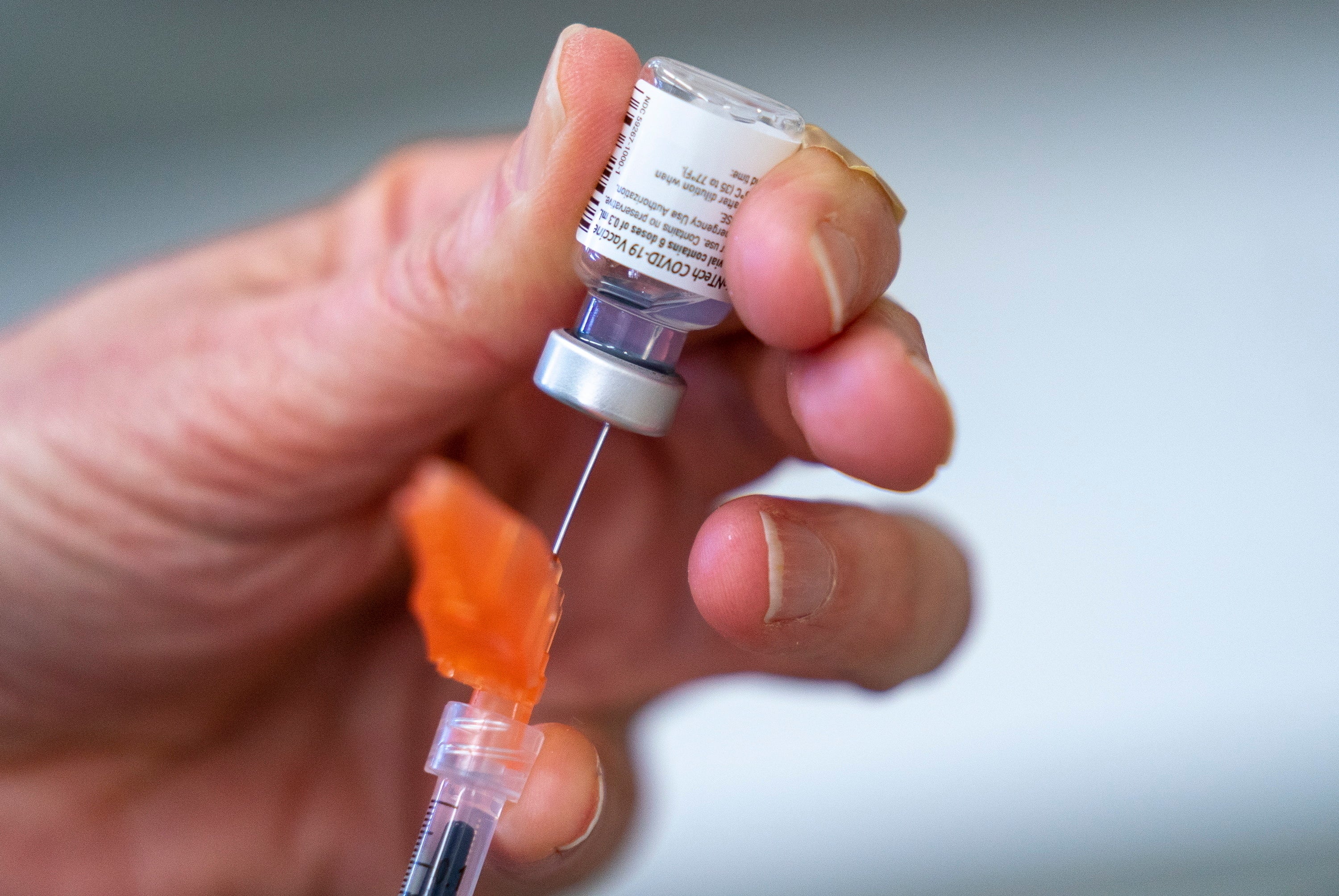
Your support makes all the difference.Canada’s health regulator approved Pfizer’s kid-size COVID-19 shot on Friday.
Health Canada authorized the shots for children ages 5 to 11. And as in the U.S., the doses will be just a third of the amount given to teens and adults.
But Canada's National Advisory Committee on Immunization has suggested that the country's provinces, which administer health care in the country, offer the two doses at least eight weeks apart.
In the U.S. 5- to 11-year-olds receive two low doses, three weeks apart, the same schedule as everyone else in the U.S. Canada had problems getting vaccines into the country early this year and delayed a second dose for adults until more supply came in, but Canadian officials say delaying a second dose provides better protection.
“A longer interval between doses leads to stronger immunity,” said Howard Njoo, the deputy public health officer of Canada.
The government agency said the vaccine is 90.7% effective in preventing COVID-19 in children and no serious side effects were identified.
“After a thorough and independent scientific review of the evidence, the Department has determined that the benefits of this vaccine for children between 5 and 11 years of age outweigh the risks,” Health Canada said in a statement.
In the U.S., the White House said Wednesday that about 10% of eligible children aged 5 to 11 have received a dose of the Pfizer COVID-19 vaccine since the U.S. approval for their age group two weeks ago.
At least 2.6 million kids have received a shot, White House COVID-19 coordinator Jeff Zients said Wednesday, with 1.7 million doses administered in the last week alone, roughly double the pace of the first week after approval.
Canada is expecting an accelerated delivery of 2.9 million child-sized doses, enough for a first shot for every child in the 5 to 11 age group. In a statement Friday, Pfizer said the vaccines would be shipped to Canada “imminently.”
Dr. Andrew Morris a professor of infectious diseases at the University of Toronto and the medical director of the Antimicrobial Stewardship Program at Sinai-University Health Network, said delaying a second dose for eight weeks probably means a lower myocarditis risk and theoretically a better immune boost. He said there are extremely rare side effects, including myocarditis, a type of heart inflammation that occasionally occurs after the second dose.