Canada getting 168K Moderna vaccine doses before year end
Prime Minister Justin Trudeau says Canada is contracted to receive up to 168,000 doses of the Moderna COVID-19 vaccine before the end of December, pending approval by Canada’s health regulator
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
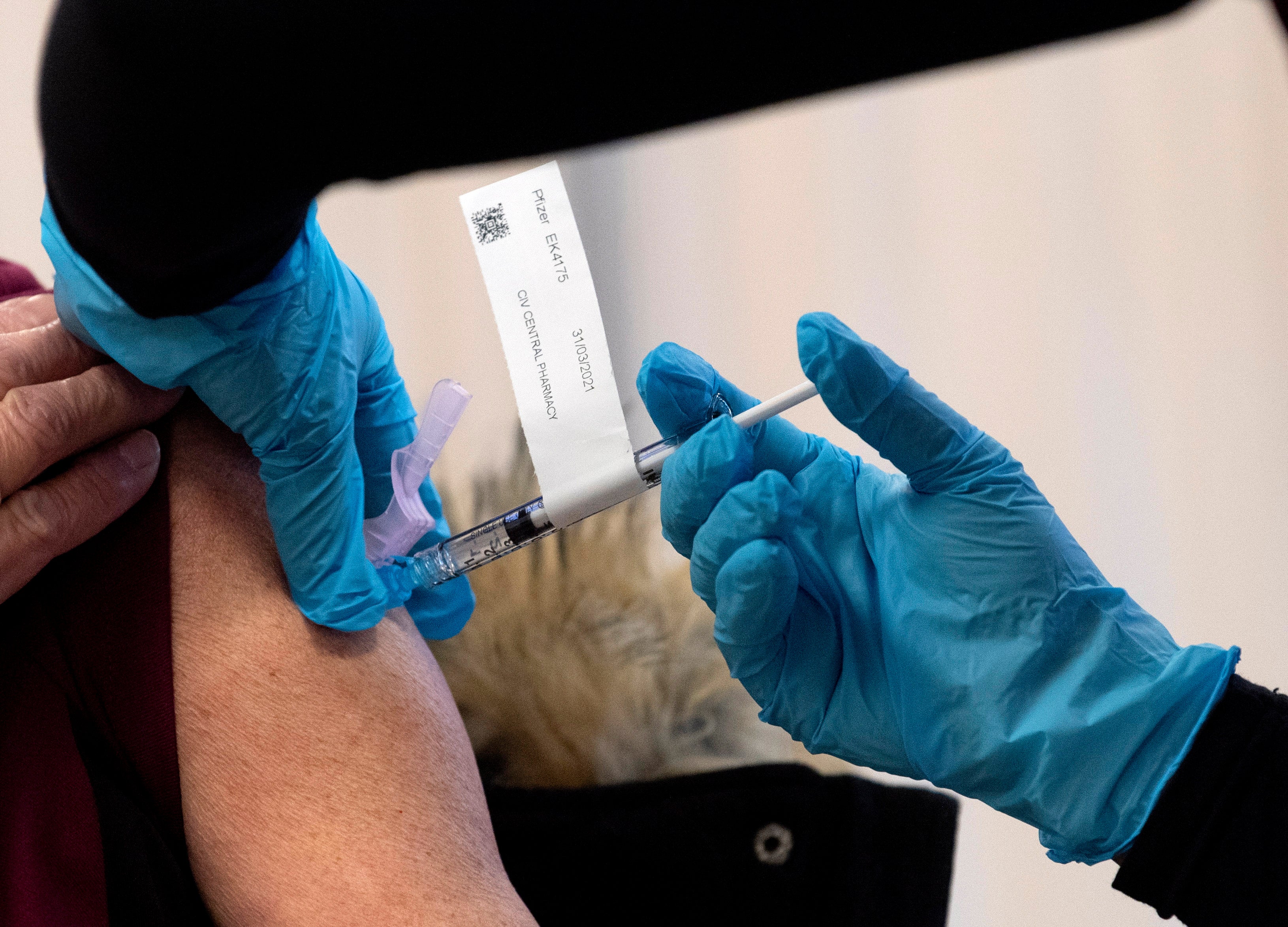
Your support makes all the difference.Prime Minister Justin Trudeau said Tuesday that Canada has contracted to receive up to 168,000 doses of the Moderna COVID-19 vaccine before the end of December, pending approval by the country's health regulator.
Trudeau said deliveries could begin within 48 hours of regulatory approval and health officials said they expect to approve use of the Moderna vaccine soon.
Canadians began receiving vaccine shots developed by Pfizer and BioNTech on Monday and Trudeau said Canada expects to receive about 200,000 doses from Pfizer next week. Canada received an initial batch of 30,000 this week.
Trudeau said they will have 70 sites ready to administer these doses next week, up from 14 sites this week.
The U.S. Food and Drug Administration said its preliminary analysis confirmed the effectiveness and safety of the vaccine developed by Moderna and the National Institutes of Health, bringing it to the cusp of U.S. authorization. A panel of outside U.S. experts is expected to vote to recommend the vaccine on Thursday, with a final FDA decision coming soon thereafter.
Moderna’s vaccine is the same type as Pfizer’s, made with the same technology. In scrutinizing early results of a 30,000-person study, the FDA found it worked just about the same.
The Moderna vaccine was more than 94% effective overall at preventing COVID-19 illness, and 86% effective in people 65 and older. The FDA uncovered no major safety issues.
Trudeau noted the Moderna vaccine does not need some of the extra special handling requirements of the one from Pfizer, including ultra cold freezers.
“That makes it a better option to ship over long distance to remote areas so doses of this vaccine will be directed to the north as well as remote and Indigenous territories,” Trudeau said.
The French-speaking province of Quebec, meanwhile is closing non-essential businesses from Christmas until at least Jan.11. Quebec Premier Francois Legault said that big box stores will be prohibited from selling any goods that are deemed non-essential. The premier is also forcing all office towers to empty starting Thursday and requiring employees to work from home until at least Jan. 11. Legault said elementary and secondary schools will close Dec. 17 and can reopen at the earliest on Jan. 11.
He said hospitals across the province are under too much pressure because of the COVID-19 pandemic to allow non-essential businesses to stay open during the holidays.
Quebec reported 1,741 COVID-19 infections on Tuesday.
Ontario, Canada's most populous province, is reporting a new single-day record of 2,275 new cases including 711 in Toronto.