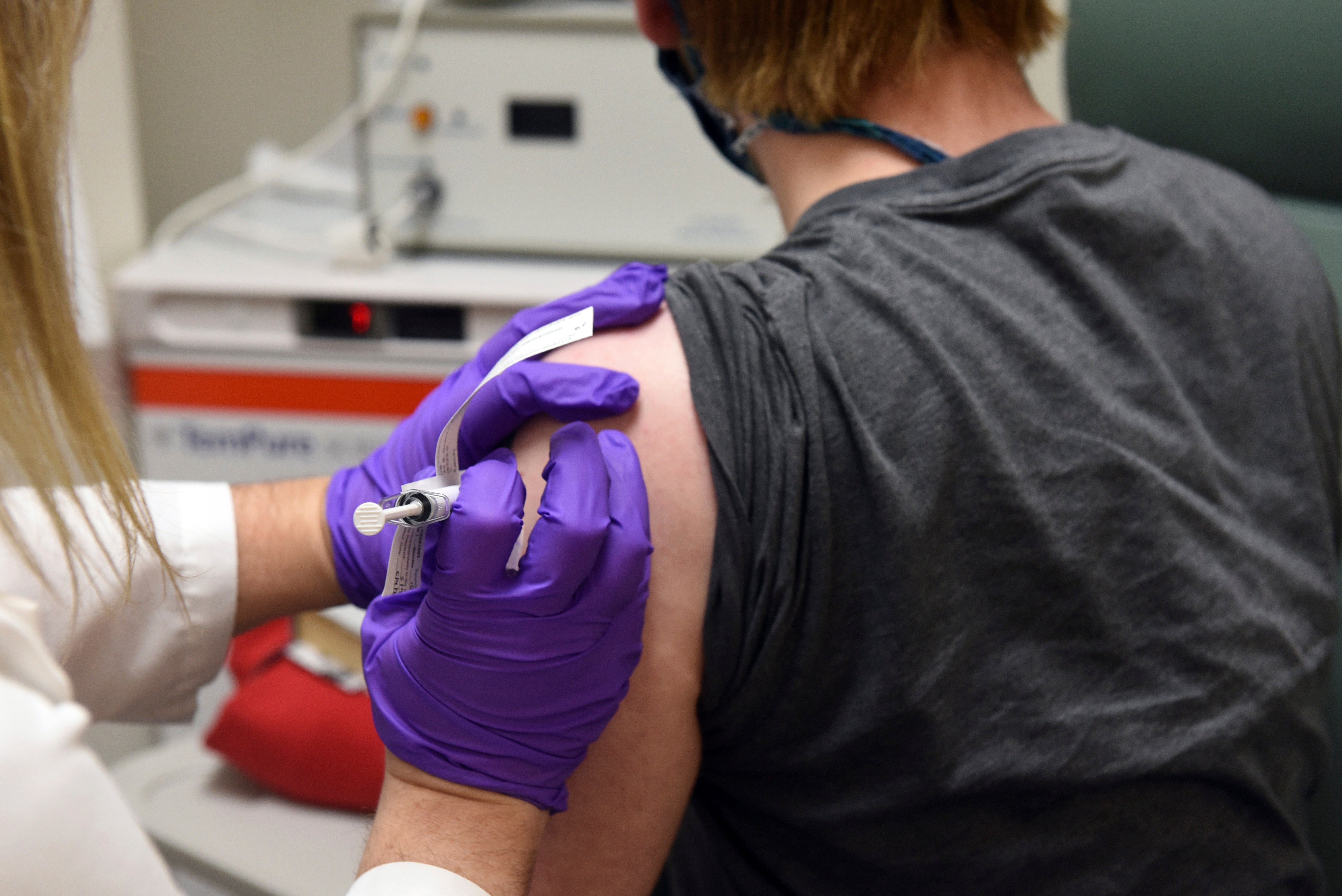
BioNTech, Pfizer ask Europe to OK vaccine for emergency use
German pharmaceutical company BioNTech and its U.S. partner Pfizer say they have submitted an application for conditional approval of their coronavirus vaccine with the European Medicines Agency
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.The German pharmaceutical company BioNTech and its U.S. partner Pfizer say they have submitted an application for conditional approval of their coronavirus vaccine with the European Medicines Agency.
The two companies said Tuesday that the submission, which occurred Monday, completes the rolling review process they initiated with the agency on Oct. 6.
The move comes a day after rival Moderna said it was asking U.S. and European regulators to allow emergency use of its COVID-19 vaccine.
BioNTech said if the vaccine, currently named BNT162b2, is approved, its use in Europe could begin before the end of 2020.
The companies said last month that clinical trials with tens of thousands of participants showed the vaccine had an efficacy rate of 95%. The success rate in particularly vulnerable older age groups was more than 94%, they said.
BioNTech and Pfizer have already submitted a request for emergency approval with the U.S. Food and Drug Administration and the U.K. regulator MHRA, as well as rolling submissions in other countries including in Australia, Canada and Japan.
“We have known since the beginning of this journey that patients are waiting, and we stand ready to ship COVID-19 vaccine doses as soon as potential authorizations will allow us,” Pfizer’s chief executive Albert Bourla said in a statement.
Germany’s science minister said Tuesday that the same safety standards are being applied in the approval process for coronavirus vaccines as for other drugs and that this would be key to gaining the widest possible public acceptance for COVID immunization.
Anja Karliczek told reporters in Berlin that the EMA will be holding a public hearing on Dec. 11 on the approval request by BioNTech and Pfizer.
She added that the vaccine will be voluntary and that authorities will work hard to inform the public about possible side effects that might be expected after immunization, such as headaches, localized pain and fever.
Marylyn Addo, a doctor at Hamburg’s UKE hospital who is involved in the trials for a rival vaccine, said the rapid development of a vaccine was the result of enormous efforts by scientists, early funding and experience from previous vaccines.
___
Follow AP’s coverage at https://apnews.com/hub/coronavirus-pandemic and h ttps://apnews.com/UnderstandingtheOutbreak