Questions over vaccine safety
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
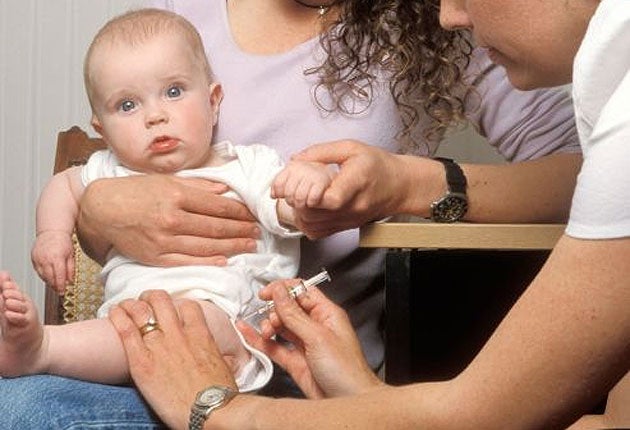
Your support makes all the difference.Health officials have been forced to withdraw 21,000 doses of the meningitis C vaccine from GP clinics around the UK after it emerged that some doses may have been contaminated with a blood-poisoning bacterium.
More than 60,000 doses of the vaccine, which is offered to all four-month-old babies, could be contaminated with the hospital-acquired infection – the Staphylococcus aureus bacterium – and a third of these had already been sent to vaccination clinics before officials became aware of the problem.
Officials within the Department of Health and the vaccine's manufacturers are believed to have known of the problem since Tuesday but only issued an emergency recall last night after being contacted about the potential contamination by The Independent.
Last night the Tory health team demanded answers about why it had taken so long to withdraw the vaccine and
said there may need to be an inquiry. It is not known how many children may have received the dose, but one official said that there have as yet been no reports of any adverse reactions.
In a statement, the Department of Health denied that any contaminated samples had entered the UK market. "Two batches have been identified and are being recalled as a purely precautionary measure. These two batches passed all routine quality testing, including a sterility test."
The revelation comes at a critical time given that many parents are still suspicious about childhood vaccinations due to the unfounded scare over the measles, mumps and rubella (MMR) vaccine which was incorrectly linked to autism in a study published over 10 years ago and since retracted.
The meningitis C vaccine, sold under the trade name Menjugate, is manufactured and packed in Italy by Novartis, a multinational pharmaceutical company. One insider told The Independent that any contamination with Staphylococcus – which is related to MRSA – would have occurred during the preparation of the vaccine in the manufacturing centre in Siena.
The company confirmed last night that two lots of the vaccine – some 61,117 doses imported into Britain – could be contaminated and that 21,301 doses have been sent to GP surgeries over the past week for the Government's childhood vaccination programme.
The Medicines and Healthcare products Regulatory Agency (MHRA) was working last night with Novartis to recall all the remaining doses of vaccine that had been sent to GPs but which had not yet been used. A spokeswoman for Novartis said that the company's press office was made aware of the problem on Monday but she could not say when the company had informed the Department of Health.
"Novartis is working with the relevant government authorities including the MHRA and the Italian Ministry of Health to recall two lots of Menjugate distributed in the UK," the spokeswoman said.
"We are investigating a sterility testing positive result from samples of one lot of aluminium hydroxide solvent which was used in the packaging of two lots of Menjugate. The solvent lot passed all release specifications. The subject result was identified during a special study," she said.
The MHRA said in a statement issued last night that it was first alerted to the problem on Tuesday. "The tested samples were of one batch of solvent used in two batches of Menjugate Kit, and were identified positive for the bacteria Staphylococcus aureus during the sterility test. They [the two samples] were not distributed to the UK market," it said.
"However, as a precaution, these two batches of Menjugate Kit which were distributed in the UK are being recalled. There is at present no evidence that these two batches of Menjugate Kit are affected," it added.
A spokeswoman for the MHRA said that, despite the recall, there was no evidence of any risk to children from the vaccine but she could not say how many babies could have already been injected with the vaccine. "The recall is purely a precautionary measure. There is no reason for UK children to be at any risk from this product. All vaccine supplied to the UK had passed the tests required for its use," she said.
Andrew Lansley, the shadow Health Secretary, said: "This is very disturbing news. We will be looking to the Government to give the fullest possible account of what's happened.
"Parents who bring their children for immunisation need to have the greatest possible confidence that the vaccines concerned are safe and their children won't come to harm. I will be looking to the Government to provide this specific assurance."
Mike Penning, a Tory health spokesman, said: "Parents take vaccines to make their children safe, not put them at risk. They will want answers as to why it took so long to withdraw the vaccine. The Government has got to come clean about its decision-making and when the Secretary of State was told what happened. We could need an independent inquiry to establish the facts."
The Menjugate vaccine – one of two distributed in the UK – was the subject of safety concerns in 2000 soon after it was introduced into Britain. However, the Medicines Control Agency said at the time that the fears were unfounded.
Pat Troop, then deputy chief medical officer, said in 2000 the meningitis C vaccine was very safe. The number of adverse reactions – such as headaches, and sore arms – were normal for this sort of vaccine, she said.
Novartis: The company behind the jab
The Swiss pharmaceutical giant Novartis is one of the largest medicine manufacturers in the world with 81,000 employees operating in 140 countries. It was formed out of the merger in 1996 of two "big pharmas", Ciba-Geigy and Sandoz. In 2006, Novartis acquired the US-based vaccine supplier Chiron Corporation. Two years earlier, Britain's Medicines and Healthcare Products Regulatory Agency seized more than 48 million doses of Fluvirin, an influenza vaccine made in Chiron's factory in Liverpool, over concerns they were contaminated. The seizure caused a major shortage of flu shots in the US market in which Chiron had a near monopoly. Nonetheless the vaccine market has remained a strong moneyspinner for Novartis. Last month the US government awarded it a $486m (£342m) contract to build a factory to produce a cell-based influenza vaccine which would be capable of churning out 150 million flu shots within six months of Washington declaring a flu pandemic. In Britain over the past two years Novartis has been trying to create a vaccine for meningitis B, which kills more than 100 children every year. Novartis, the Health Protection Agency and the Oxford Vaccine Group have been carrying out a joint trial on three different strains of meningitis B with positive results. Novartis expects to apply for a licence next year and hopes to have it on the market by 2011. Currently 10 per cent of the children with meningitis B die and 15 per cent of those who survive are left with severe disabilities.
Jerome Taylor
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
0Comments