Another US kids' swine flu vaccine recalled
Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
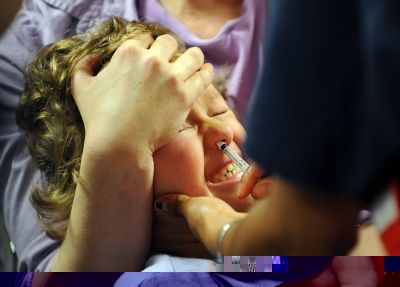
Your support makes all the difference.A week after 800,000 doses of children's swine flu vaccine were recalled in the United States for losing their potency, a second vaccine maker recalled its A(H1N1) nasal spray for the same reason.
MedImmune announced late Tuesday that it was "voluntarily recalling unused doses of 13 specific lots of influenza A(H1N1) 2009 Monovalent Vaccine Live, Intranasal due to a slight decrease in potency.
"There is no safety concern with the lots that are being recalled," it added in a statement.
The recall involves doses with best-before dates between January 19 and January 26, 2010.
MedImmune's nasal spray vaccine was the first to become available in the United States, but can only be administered to children older than two and healthy adults up to age 50.
According to Food and Drug Administration spokeswoman Karen Riley, the recall involves 3,000 doses of the nasal spray still at a central distribution center and an unknown number at healthcare facilities or with health care authorities in various states.
"Given the intense demand in October and November, we don't expect there is much left with healthcare facilities or in the states," said Riley.
Children and adults who had the nasal spray vaccine in the first months of the vaccination campaign in the United States do not need to be vaccinated again, MedImmune said.
Last week, Sanofi Pasteur recalled 800,000 doses of injectable swine flu vaccine for children after routine tests showed they had lost potency.
Like the MedImmune vaccine that was recalled Tuesday, Sanofi Pasteur said its treatment lost minimal strength and that children already inoculated with vaccine from the recalled lots would not require a second round of vaccinations.
kdz/oh
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments