What the microbes in your gut can tell you about your general health
Some microbes increase across a range of diseases, so detection of them could give an early heads up that something’s not right

Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.The number of studies that have found a link between a disease and a specific gut microbiome composition seems to be ever increasing.
Until recently, almost all these studies have looked at single diseases in isolation. But most people tend to have more than one health complaint at a time – “comorbidities”, in medical parlance.
For our latest study, published in Nature Communications, we looked at the gut microbe composition across a range of diseases. What we found surprised us. The kind of microbes (such as Enterobacteriaceae) that increased in one disease, increased in pretty much all 38 diseases studied. Also, some microbes that might be considered to be “healthy gut microbes”, were reduced in all 38 diseases studied.
We used data from the TwinsUK cohort, a unique group of older British twins who have shared their health history, and many biological samples, for over 25 years. They are volunteers who, like all of us who have lived a while, have gathered health problems over time – 96% of the 2,700 who have donated stool samples have one or more health problems.
The most striking finding from our analysis was that the microbes weren’t specific to individual diseases, but rather to the state of general health. From a biological perspective, this makes sense. The environment that each bug likes is quite specific; anything that alters it, even slightly, means some sensitive bugs won’t survive.
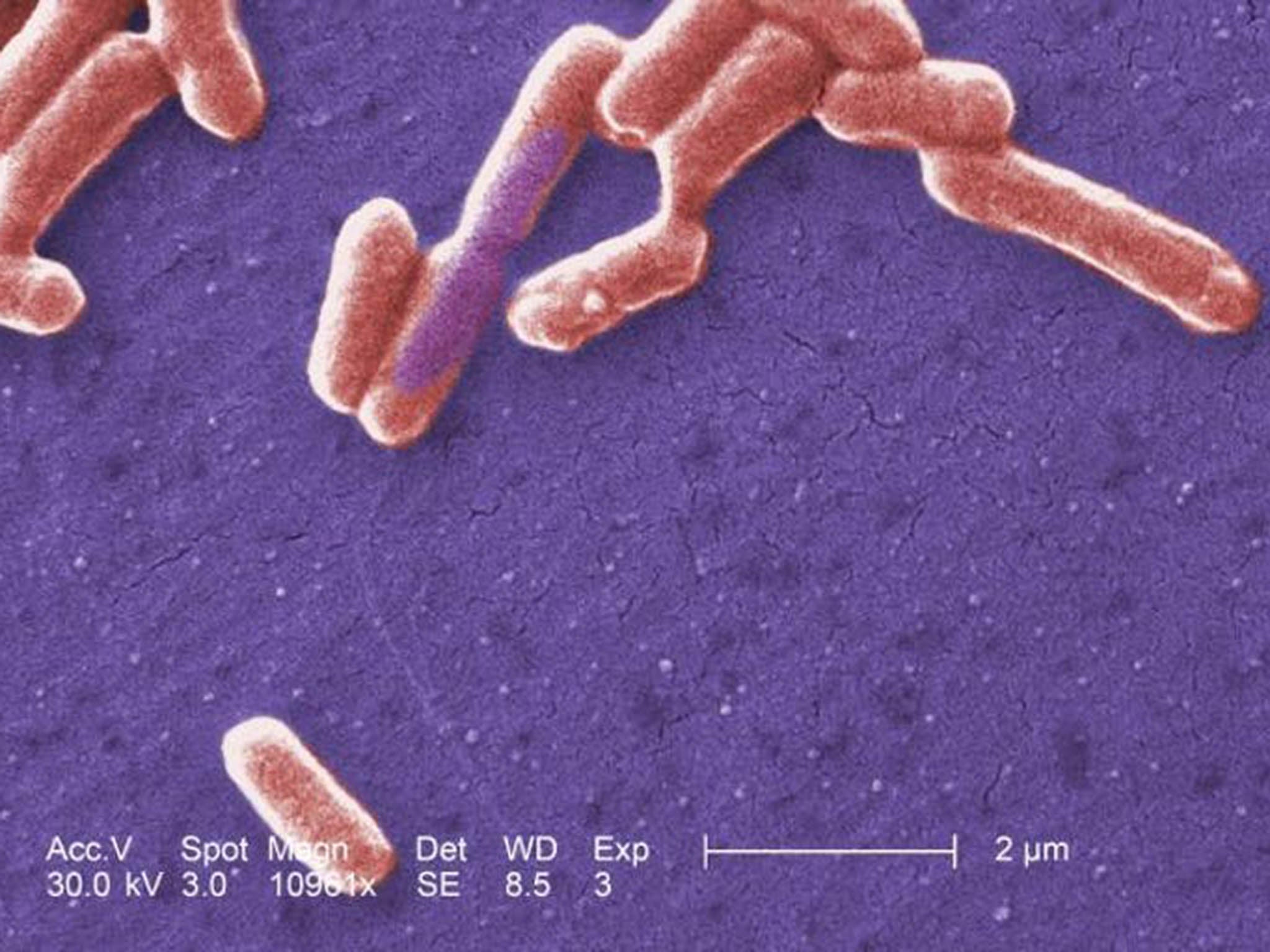
For example, the colon is a surprisingly low-oxygen (anaerobic) environment. Many illnesses lead to low level inflammation, which means that tiny blood vessels open and white cells creep out into tissues, including in the gut. White cells use oxygen as a weapon, so oxygen levels in the colon rise. This can be toxic to the normal gut bacteria, which evolved for strictly anaerobic conditions. An example is the friendly (yet frightening sounding) bug Faecalibacterium Prausnitzii, which is wiped out in the presence of almost any disease.
The knock-on effect the loss of these friendly microbes has on a person’s health is not yet known. They may simply be markers of good health, or they may actively contribute to good health. If they do contribute to good health, doctors will need to intervene early in the disease process to keep the friendly bugs alive. This might involve taking prebiotics (food for the friendly bugs) and probiotics, side by side.
In the future, researchers may even find a way to isolate your healthy gut bacteria and grow them outside your gut. Once enough have been grown, they could be reintroduced to your gut to boost your health. A personalised combination of healthy gut bacteria may be more likely to survive in your gut than a random implant of any good bacteria.
Care in the community
A family of bacteria that increased in all the diseases we looked at was Enterobacteriaceae. These bacteria are adapted to survive in higher oxygen environments than the normal colon, and they include bacteria, such as E coli, that can make you really ill. They also harbour high numbers of antibiotic-resistant genes.
Bacteria can pass special genes between each other (horizontal gene transfer) to survive an antibiotic onslaught. So if it turns out that bacteria which carries those genes are also found in people with multiple diseases, then it makes a difference to how we deliver safe, effective care for patients while maintaining infection control. For example, putting a bunch of vulnerable people together in a hospital is likely to create more opportunities for virulent strains of bacteria to evolve. We might need to invest more in treating people safely in their own homes.
Bug sensors and bug census
Our findings suggest that we could all benefit from being more aware of exactly what we are carrying inside us. Specifically, it suggests two things.
One, bugs are good sensors of our general health. So, in the future, we might want to consider over-the-counter poo tests to monitor our overall health. An early warning, such as a dip in anaerobic bugs, could help us to head things off at the pass. Subsequent tests could tell us if any of the action we have taken is working. If not, we can change tack.
Two, we should take a regular census of the bugs inside us, especially those associated with antibiotic resistant genes. The science is still in its infancy, but knowing where we are with these guys may help preserve antibiotics for when we really need them.
Claire Steves is a clinical senior lecturer at King’s College London. This article first appeared on The Conversation (theconversation.com)
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments