A killer fungus is spreading through UK hospitals – here's what you need to know about Candida auris
The spread of a multidrug-resistant fungal infection is rapid and alarming

Your support helps us to tell the story
From reproductive rights to climate change to Big Tech, The Independent is on the ground when the story is developing. Whether it's investigating the financials of Elon Musk's pro-Trump PAC or producing our latest documentary, 'The A Word', which shines a light on the American women fighting for reproductive rights, we know how important it is to parse out the facts from the messaging.
At such a critical moment in US history, we need reporters on the ground. Your donation allows us to keep sending journalists to speak to both sides of the story.
The Independent is trusted by Americans across the entire political spectrum. And unlike many other quality news outlets, we choose not to lock Americans out of our reporting and analysis with paywalls. We believe quality journalism should be available to everyone, paid for by those who can afford it.
Your support makes all the difference.At least 20 NHS Trust hospitals have been hit by a drug-resistant fungus, known as Candida auris. So far, 200 people have been contaminated or infected with the fungus, which can cause potentially deadly complications.
The fungus, which is able to live on the skin or inside the body, was first discovered in Japan in 2009. Since then it has been found in at least 15 countries, including the UK, where the first case was identified in 2013.
While many new diseases generate both dismissive “not another one” attitudes and “the end is nigh” hysteria, fungal diseases rarely cause much of a ripple in public health, compared to viruses or bacteria. But there are several reasons C. auris represents a significant concern for those trying to keep the UK population healthy.
The early indications are that many of the infections it causes are life threatening – and it has characteristics that raise serious concerns over the short and long-term effectiveness of antifungal drugs. Of the more than 200 cases in the UK since 2016, more than 10 per cent have been systemic bloodstream infections, which are typically the most serious kind of fungal infection.
These systemic infections, known as candidemia or fungemia, are notoriously difficult to diagnose and treat. The persistent, localised and high-mortality cases that have made up the bulk of the reported infections across the world are probably hospital-acquired.
Multidrug-resistant strain
Being acquired in the hospital puts the most vulnerable patients right in the way of C. auris. Patients with weakened immune systems and those requiring treatment for other diseases are the ones most likely to get infections.
Even more troubling, some strains of the fungus appear to have natural resistance to all three classes of antifungal drugs. There are limited antifungal options in the clinic, and a hospital transmissible multidrug-resistant strain is quite threatening. If C. auris is able to persist in hospitals, then drug-resistant strains may repeatedly emerge, and hospitals might become breeding grounds for the worst strains.
Unprecedented spread
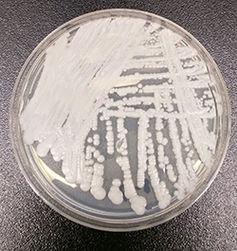
Compared with many other fungi, the emergence of this pathogen is occurring at a surprising pace. C. auris was first documented after it was found in the ear of a patient in a hospital in Tokyo in 2009. However, recent global outbreaks, mostly since 2013, appear to stem from few a few places, suggesting that the fungus is either a rapidly spreading novel pathogen or is being driven by changes in clinical conditions. Although there is good evidence for the rapid spread of fungal diseases of animals and plants, there is virtually no precedent for a rapidly spreading fungal disease of humans.
Most fungal diseases in humans are environmentally acquired and their spread tracks events in the environment rather than the clinic. Although the sudden emergence of fungal diseases associated with the global Aids epidemic appeared very rapid, the fungi themselves were already present.
It remains to be determined what the driving factor is behind the rapid emergence of C. auris, but health agencies across the world are on the alert for new outbreaks and research is very active.
Finally, because it is a very recently discovered fungal disease, we know very little about its abilities and vulnerabilities. Studies so far have found that C. auris may display traits associated with virulence similar to other fungi such as biofilm formation (forming sticky matrices on surfaces) and the production of protein-degrading enzymes – but, because scientists are only just beginning work with this fungus, there are a lot of unknowns.
It is not yet clear which, if any, specific traits enable C. auris to invade hosts, or if strains differ in virulence. Similarly, although the evidence supports C. auris being resistant to antifungals, the mechanisms of resistance remain unknown.
In the bigger picture, key questions remain. Where is C. auris from? Are the clinical strains different from strains outside the clinic? What is the route of transmission, if any, within and between hospitals? These unknowns make C. auris challenging for professionals and disconcerting for the public.
Daniel Henk is a lecturer at the University of Bath. This article was originally published on The Conversation (www.theconversation.com)
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments