‘Green gold’: Could we feed the world sustainably with microalgae?
Microalgae are massively abundant in our seas and act as biomass factories, by harnessing their power we may be able to alleviate food-security issues around the world, writes Carole Anne Llewellyn

As a young child in the mid-1960s, my days were spent living an idyllic rural life on a dairy farm in the village of Lewdown in the heart of Devon. I recall many happy days exploring the glorious countryside, living a life in balance with nature and the environment – or at least, that’s how it felt.
But I also remember the ever-present slurry pit full of manure down at the end of our cowshed. It wasn’t fenced off, and my mum would remind me on regular occasions that to stray too close could mean death by drowning in what was, in essence, an enormous vat of smelly cow pats. As a five-year-old, I stayed well clear.
What we didn’t know then was that this pit of farm manure posed not only a hazard to me, but to our environment. Manure, which is often returned to the land as a nutrient fertiliser without consideration of its wider impacts, releases greenhouse gases including methane, carbon dioxide and nitrous oxide, and other harmful nitrogenous gases such as ammonia. It can also lead to nitrogen-rich run-off into water courses, polluting rivers, lakes and coastlines – with knock-on effects on fish mortality and tourism.
In short, what I thought was an idyllic childhood, living on a farm in balance with nature, wasn’t quite that. Subsequently, as a bioscientist, I’ve spent much of my life researching microorganisms that can help maintain a healthy planet. Nearly 60 years later, I find myself leading a pioneering Europe-wide project dedicated to transforming potentially harmful waste into something positive. In the process, we can help to build a circular economy that regenerates nature and keeps materials in circulation. And at the centre of this work are some remarkable microscopic organisms – our “green gold”.

Jewels of nature
We all know how important trees are in terms of sequestering carbon, yet we tend to overlook the two-thirds of our planet that is covered by water. Our seas and oceans are filled with organisms that are equally vital to the Earth’s life cycles, yet because they are individually less visible to the naked eye than land plants, we largely ignore them.
Microalgae – not to be confused with macroalgae (seaweeds) – are massively abundant in our seas, freshwater lakes and rivers. These tiny organisms are important “primary producers” on our planet, acting as biomass factories. They use sunlight through the process of photosynthesis to convert inorganic molecules (carbon dioxide, nutrients and water) into proteins, fats and carbohydrates, plus a host of other organic compounds that help them grow and survive. These tiny microorganisms support all life in our oceans and, with their high turnover rates, contribute to around 50 per cent of the planet’s primary production.
There are literally hundreds of thousands of species of microalgae. A commonly occurring group are the diatoms, of which there are an estimated 20,000 species. With beautifully intricate, snowflake-like cell walls made of glass, diatoms are true jewels of nature. Another common group are the coccolithophores, covered in elaborate, frisbee-like calcium carbonate chalk plates. During the Cretaceous period, which ended 66 million years ago, enormous blooms of coccolithophores formed the white cliffs of Dover.
By recycling unwanted nitrogen into something useful, we can prevent it escaping into the atmosphere and into waterways, thereby reducing pollution to both land and atmosphere
As microalgae do not have roots, leaves and stems, they can use carbon dioxide and nutrients more efficiently than land plants, enabling them to grow more rapidly. They can be relatively easily cultivated and harvested to produce biomass crops – algaculture – which can be used as food or for bioenergy. Algal biomass also contains a wide range of useful molecules that can be used in bioplastics, biofuel, health products, cosmetics and food ingredients.
My growing appreciation of these fascinating microorganisms, with their amazing ability to grow on waste nutrients and produce something useful, inspired me to want to help address the twin global challenges of sustainability and environmental protection. Using nature’s green gold to clean up waste nutrients while also producing sustainable feeds and other products seemed to me a no-brainer.
Back in the 70s, I recall my A-level biology teacher, Mr Montague, introducing us to the carbon and nitrogen cycles and explaining how important the balance of each of these cycles is to life on our planet. I even remember him talking about the greenhouse effect and temperature rise. But we didn’t realise back then just how severe the threat of carbon dioxide-related climate change was – or how nitrogen would emerge as a major contributor to the complex environmental challenges we face today.
Towards a circular economy
To have any hope of meeting our global climate change targets and achieving a sustainable equilibrium, we need to work towards a circular economy that eliminates waste and pollution, keeps materials in circulation and regenerates nature. This must replace our existing linear “use and discard” model which has led to unbalanced nutrient cycles.
In response to this, farmers, the food industry and waste-water companies are increasingly turning to anaerobic digestion (AD) to process their waste. AD is a natural process in which bacteria in large tanks called digestors feed on organic waste – sewage, food waste, farm manure and other agricultural waste – to produce a biogas, rich in carbon and hydrogen, that can be captured and used to generate renewable electricity and heat.

The nitrogen component of the organic waste is retained in a thick liquid called digestate, which can be returned to the land by farmers as a naturally produced fertiliser – preferable to synthetic fertilisers produced using energy-intensive and CO2-emitting processes. However, as the AD industry has expanded, so the increased production and returning of digestate to the land poses a risk of nutrient pollution.
As a result, many areas in the United Kingdom and Europe are now restricted by the Nitrate Directive and nitrate vulnerable zone (NVZ) legislation, introduced to prevent pollution through excessive use of nitrogen returned to the land. Currently, 55 per cent of land in England is designated an NVZ, while the entirety of Wales is in the process of becoming another such zone.
One way of overcoming this regulatory challenge is through the use of microalgae. And so, in 2017, our Europe-wide, circular economy project called ALG-AD was born. The ultimate goal is to convert nitrogen that poses a risk to the environment into microalgae that can be used in sustainable animal feed, replacing existing, highly resource-intensive sources of feed in the process. Using funding from the Interreg North-West Europe programme, Swansea University partnered with 10 other organisations throughout north-west Europe – a densely populated and intensely agricultural area that is particularly vulnerable to nitrate pollution of groundwater. The whole of Belgium, Germany, the Netherlands and Denmark are also already designated NVZs.
By recycling unwanted nitrogen into something useful, we can prevent it escaping into the atmosphere and into waterways, thereby reducing pollution to both land and atmosphere. The microalgae naturally convert the nitrogen into protein and other nutritional molecules which can be used back in the food chain. Five years on from the project’s launch, we have already shown that such a circular economy solution is workable on an industrial scale.
A new source of protein
The projected growth of the planet’s population over the next half-century means global food production is expected to increase by at least 50 per cent. We are also all being encouraged to reduce consumption of meat protein to reduce greenhouse gas emissions and deforestation. New sources of protein are therefore a top priority, and microalgae are strong contenders. Companies such as Nestle are already researching microalgae as an alternative source of protein, both as animal feed and food for humans.
While the microalgal production industry is still in its infancy, the ability to produce a new source of protein without the issues associated with meat and soya is very attractive. Furthermore, being able to cultivate microalgae close to where they will be used by farmers in animal feed offers another distinct advantage.
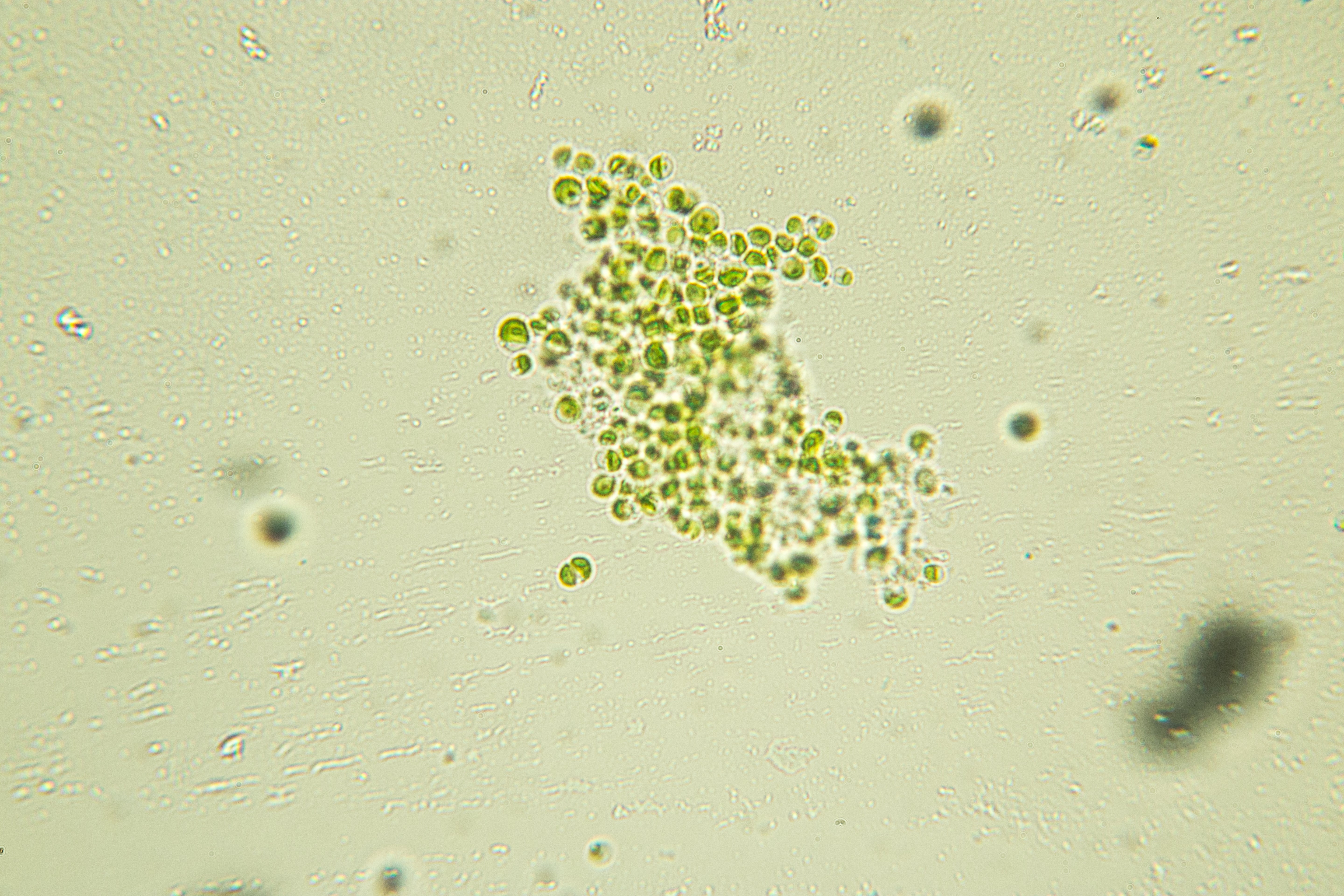
A big challenge for our European project has been to test this technology for development at full working scale. We have therefore worked directly with the AD industry as it processes food and farm waste, providing us with industrially produced nitrogen (in digestate) to cultivate our microalgae.
In the UK, just 30 miles from the Devon farm on which I lived as a child, we have built a pilot “algae-AD” facility at an AD company sited next to Langage Dairy Farm. Langage-AD has the capacity to process 20,000 tonnes of food waste a year, producing biomethane that generates heat and electricity. We were provided with a large, heated greenhouse situated right next to where the waste is processed. This was the ideal location for our “algal photobioreactor”, a series of vertical see-through tubes in which microalgae are grown in an aqueous medium containing nutrients that are exposed to both daylight and artificial light.
Two sister photobioreactor facilities have been built in Brittany in France and Ghent in Belgium. All partners have undertaken in-depth studies to determine how to best process the digestate and optimise nutrient uptake. Too much and we found that our microalgae didn’t like it; too little and not much happened.
Promisingly, we have found that microalgae grown on digestate are richer in protein compared with microalgae grown on more typically-used inorganic nutrients, with protein levels reaching up to around 80 per cent of the total biomass produced. This is well over double the amount of protein contained in meat and soya products. In a world where there is an increasing protein shortage and alternatives to meat are sought, this is a real bonus.
Our project has already demonstrated that microalgae have strong potential in helping reduce food security-related issues such as land scarcity, climate change and inefficient and unsustainable fertilise
Currently, around 75 per cent of the world’s soya crop is used as a source of protein in animal feed. As with beef production, soya production has come under scrutiny for its role in deforestation, particularly in Brazil and Argentina. In addition, the transportation of soya across the globe generates a huge carbon footprint. To top it all, transporting soya to high agricultural areas disturbs the global balance of nitrogen, leading to nutrient hotspots and an increase in NVZs.
Our studies have confirmed the potential of microalgae as a protein source to supplement and replace soya protein. However, the scale of microalgal cultivation is currently not big enough to make a significant impact on soya markets. Therefore, our real-life feed trial experiments have so far concentrated on testing microalgae as a food supplement, to improve the health of piglets and of fish. But we know that the market for algae-based animal feed and ingredients is set to grow rapidly.
Rolling out these novel biotechnologies
To date in the UK, we have focused on two commonly occurring freshwater species of green microalgae, Chlorella vulgaris and Scenedesmus obliquus (both from the division Chlorophyta). Both species contain good levels of proteins and a host of molecules with beneficial properties for health, which we are still exploring.
But yet another amazing thing about microalgae is their diversity. There are tens of thousands of other species with a breathtaking variety of form and function, still waiting to be explored.
Now, supported by the groundwork of our research, it is up to pioneering businesses, regulators and investors to work together to enable the roll-out of these novel biotechnologies more widely. As we move to a society and economy more circular than linear, which uses its waste while preventing environmental contamination, it seems that microalgae will become more familiar to us all in one form or another.

Our project has already demonstrated that microalgae have strong potential in helping reduce food security-related issues such as land scarcity, climate change and inefficient and unsustainable fertiliser usage, as well as associated nutrient leakage and water pollution. In so doing, they can be used to raise environmental standards in Europe and throughout the world. Indeed, our work supports the recently announced European Green Deal, promoting the circular economy and protection of nature, and the new Common Agricultural Policy with its strong emphasis on environment-friendly farming practices and agro-ecology.
However, it is still relatively early days. As with any waste-related technology, legislation and regulation needs to be carefully considered. For now, the simplest way forward is to use anaerobically digested vegetable-based waste rather than animal-based waste, thereby eliminating the possibility of any animal waste or animal contamination passing back into the food chain.
We would also like to further increase the uptake of digestate into the algae and, like any new and developing technology, we need to balance up the cost and overall environmental benefits. To achieve this, we are gathering results from across the partnership and consolidating our data for use in life cycle analysis. This will also enable interested farmers, food producers and other industries to decide if the technology is for them, and what they might best achieve according to their particular needs.
Another way microalgae can be used to help in agriculture is as biostimulants – natural products that, when applied in small quantities, enhance nutrition uptake and improve stress tolerance, thus reducing the need for chemical fertilisers. We are also delving further into the many other valuable components within microalgal cells, including molecules that have benefits as human and animal immune modulators, anti-inflammatories and antivirals. The full benefits of microalgae to produce new products are just waiting to be reaped.
Ironically, throughout my working life, I didn’t exactly heed the advice of my mum all those years ago, to stay away from the dangerous mix of nutrients that was brewing in the manure pit at the end of our cowshed. But I would like to think, in not doing so, that I have been part of a revolution in the way we regard and treat waste, ensuring that the valuable nutrients in cow manure and other organic waste can increasingly be used for the benefit of us, and our planet.
Carole Anne Llewellyn is a professor in applied aquatic bioscience at Swansea University. This article was first published in The Conversation.




Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments