Viruses that help: Is bacteriophage therapy the future of medicine?
If you had the right bacteriophage it could be used to treat an infection. But bafflingly, until last week, I had never even heard of this therapy, says Berenice Langdon

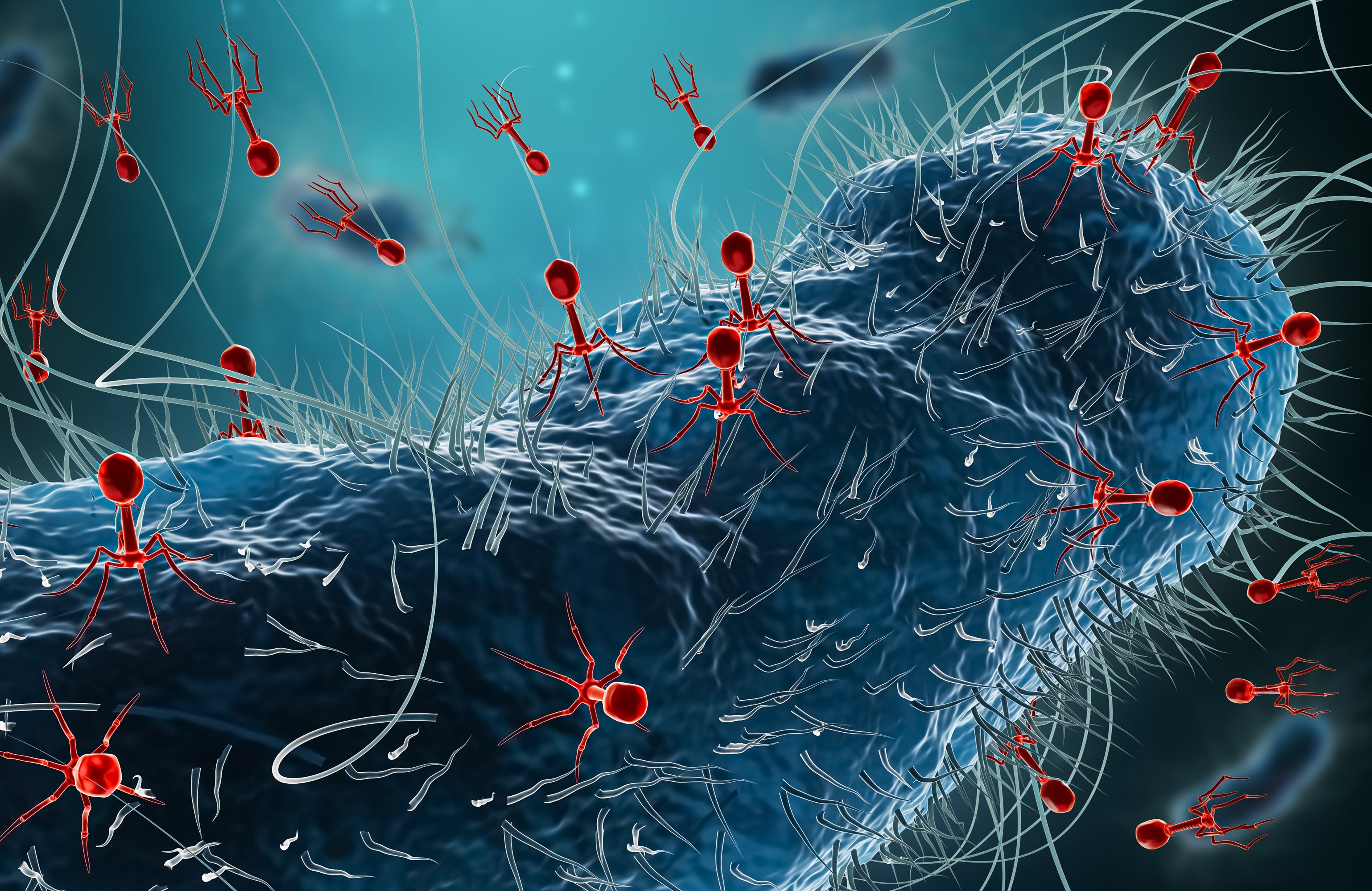
We develop phage cocktails as precision medicines to target and destroy specific harmful bacteria.’’ This is the mission statement of BiomX, a biotech company. Bacteriophage cocktails for treating bacterial infections? It sounds exotic, a bit James Bondy. But it has enough grains of truth in it to get me excited.
As far as I knew, bacteriophages were a medically unimportant group of viruses that only infect bacteria. They do this by injecting their genome through the cell wall, as viruses always do, like wasps injecting their eggs into living caterpillars. The phage then forces the bacteria to suspend all of its normal functions and mass-produce bacteriophages. The bacteria continues to do this until it explodes (or lyses, as the scientific term has it), killing itself and releasing the bacteriophages, which then continue the cycle by spreading and infecting more bacteria.
The idea of bacteriophage therapy clearly has merit. If you had the right bacteriophage, maybe it could be used to treat an infection. But bafflingly, until last week, I had never even heard of bacteriophage therapy. I was not taught about it at medical school, never read about it in any microbiology textbook and have never seen it used. Why?
Bacteriophage therapy research appears to have had a chequered history. Discovered in 1915 and successfully used as therapy almost straight away during a cavalry squadron dysentery outbreak near Paris, bacteriophages were then rubbished in a detailed scientific review in 1934. The review questioned the very concept of bacteriophages and suggested the effect was caused by enzymes. The medical community in the west lost confidence in the idea, bacteriophage therapy was overtaken by antibiotics in the 194s, and then mainly forgotten.
Except in two laboratories, the Tbilisi Institute in Georgia and the Hirszfeld Institute in Poland, where they have continued to research and develop bacteriophage therapy and use it, quite routinely, on patients.
Because of this ongoing use, particularly in Georgia, bacteriophage therapy has caused intermittent interest in the west ever since. There have been reviews and summaries published more or less every decade in well-respected journals such as Nature and Microbiology, each one recapping and repeating the (very interesting) history of bacteriophages and then giving a round up of any recent research in a kind of “where are we now” sort of way. But the answer each time is “not a lot different from the last time”. Because there are hardly any actual trials in the west. No one is trialling the therapy. Why?
We might be held back by something very simple. A difficulty in making money out of the technology. And this means pharmaceutical companies can’t afford to invest in it. You can’t patent phages for one thing, they are found naturally and abundantly in nature. Bacteriophages are cheap and easy to obtain and could even undermine antibiotic sales.
There is another distinctive quality of phage therapy that may be holding research back, and that is the concept of individualised therapy. It’s true that you can theoretically have bacteriophage cocktails, collections of six to eight phages which can be developed for use in large numbers of patients as a ready to use, off-the-peg treatment suitable for a large double-blind trial.
On the plus side, bacteriophage are super safe. They are all around us in the environment, after all. They only attack bacteria, they constitutionally can’t attack human cells
But the key joy of bacteriophage therapy is that it can be developed for individual patients. Specific harmful bacteria can be isolated from a patient, say in ICU, with an antibiotic resistant infection, and a perfect bacteriophage match identified. The patient can be treated with their particular bacteriophage which lyse the bacteria and cure the infection within hours. However, individualised therapy doesn’t fit very well into the large trials that we prefer as evidence in the west. The best we can say in each of these patient cures is that it’s a great case example, but it doesn’t properly prove anything.
There is another problem: resistance. The bacteria are constantly trying to find ways round the bacteriophage and the bacteriophages seek ways to get round the bacteria. Any bacteria resistant to a therapeutic phage will have an advantage and multiply. Of course, there are still plenty of other phages that can attack the resistant bacteria, they just have to be identified and given to the patient. But it doesn’t look good in trials. It looks like a failure.
On the plus side bacteriophage are super safe. They are all around us in the environment, after all. They only attack bacteria, they constitutionally can’t attack human cells. And they only attack specific bacteria, leaving our microbiome, our friendly bacteria, completely intact. Over the past 100 years. bacteriophages have been administered orally, intravenously, intranasally, by aerosol and topically without any ill effects. We know they are safe.

So what’s not to like? Why aren’t we using bacteriophages in our hospitals for patients in ICU with antibiotic resistant infections right now? We all know antibiotic resistance is a growing problem. Why aren’t bacteriophage products in our hospitals and pharmacies, treating the sickest members of our communities?
It turns out they are, in China. A report just published (in November 2021), Phage therapy for secondary bacterial infections with Covid-19, describes a group of eight Covid patients. They are in a Shanghai hospital ICU, during March 2020, with secondary bacterial infection, a serious antibiotic-resistant infection with a bacteria known as Carbapenem ResistantAcinetobacter baumannii (Crab). The paper describes an almost casual familiarity with bacteriophage lab technology advising that “ready-to-use phage vials are routinely prepared by using their original host bacteria in the normal phage laboratory and packed in an approved manufacturing practice plant”.
For customised phage therapy “an established library is transferred to a dedicated biosafety level (BSL) 2 lab where phage screening and evaluation are performed under BSL-3 PPE conditions. After obtaining the phage susceptibility result, phages with optimal lyse characteristics are selected and corresponding vials are transferred to the phage lab for killing-efficiency inspection.”
It’s clear that antibiotic resistance is growing, but beyond a lot of talk I don’t see pharma and investors really stepping up
Apart from the chilling “killing-efficiency” the description sounds like a surprisingly relaxed and efficient process. They must have been developing and trying those treatments within the hospital under BSL-3 conditions at a time of unprecedented stress and fear. Did anyone get authorisation or permission? Did any of the patients survive?
We don’t know. The paper simply states that “treatment with a two-phage cocktail was associated with reduced Crab bacterial burdens in all cases”.
These sorts of casual outcome measures and lack of controls give bacteriophages a bad name among western scientists. This is surely a pity. There is so much potential here for a safe and effective therapy you can almost touch it. Imagine the applications for all sorts of antibiotic-resistant pathogens, not just in ICU but in cystic fibrosis, TB, sexual health, skin related conditions, dysentery in developing countries; you name any bacterial pathogen and there is potential for bacteriophages.
Do we have labs in the UK that can rapidly identify therapeutic phages and use them for clinical patients like they did in Shanghai? How come Shanghai can do it and we can’t? Do we have banks? What do they mean by established library? An established phage library? Have we got one? We do have a bank, in Salisbury, run by Public Health England. It’s called The National Collection of Type Bacterial Cultures and it’s the oldest collection in the world, established in 1920. It contains 100 phages collected between 1950-1992, not exactly an active collection.
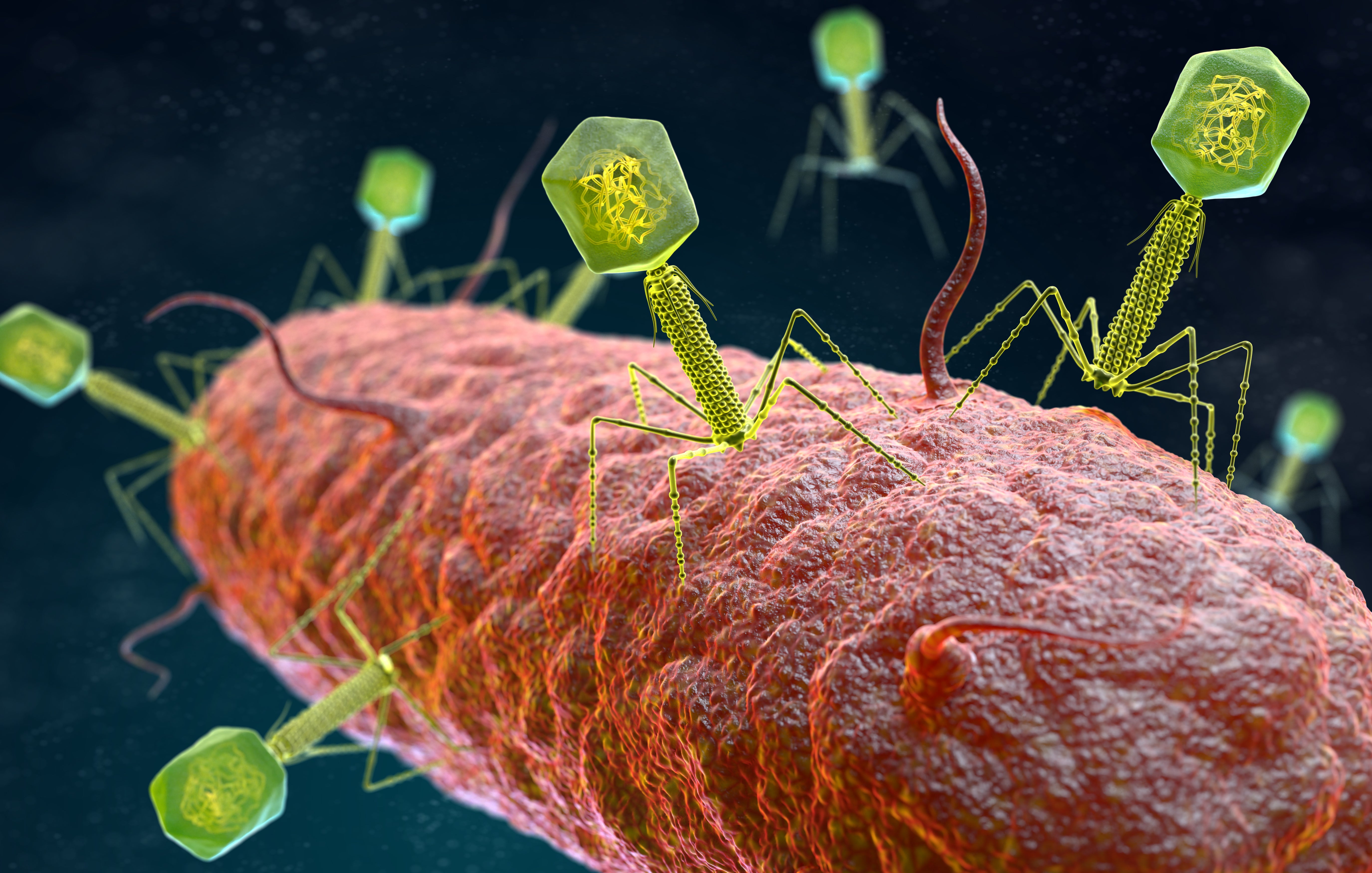
Other countries are also now setting up phage banks, for example in Israel, Belgium, the US, Germany, and South Korea as well as Georgia and Poland. Banks are useful to speed up the matching process, but in fact bacteriophages can be isolated from any environmental source that contains the target pathogen. The richest sources for bacteriophages, a gold mine really, are found in untreated hospital sewage systems.
It’s not even that hard to isolate phages. After all, Felix d’Herelle figured it out in 1915, self-treated to check safety, developed effective therapies for various infections and even wrote a handbook on how to do it. There is a modern handbook now: Bacteriophage Therapeutics, from lab to clinical practice, describing to a modern researcher exactly how to do it.
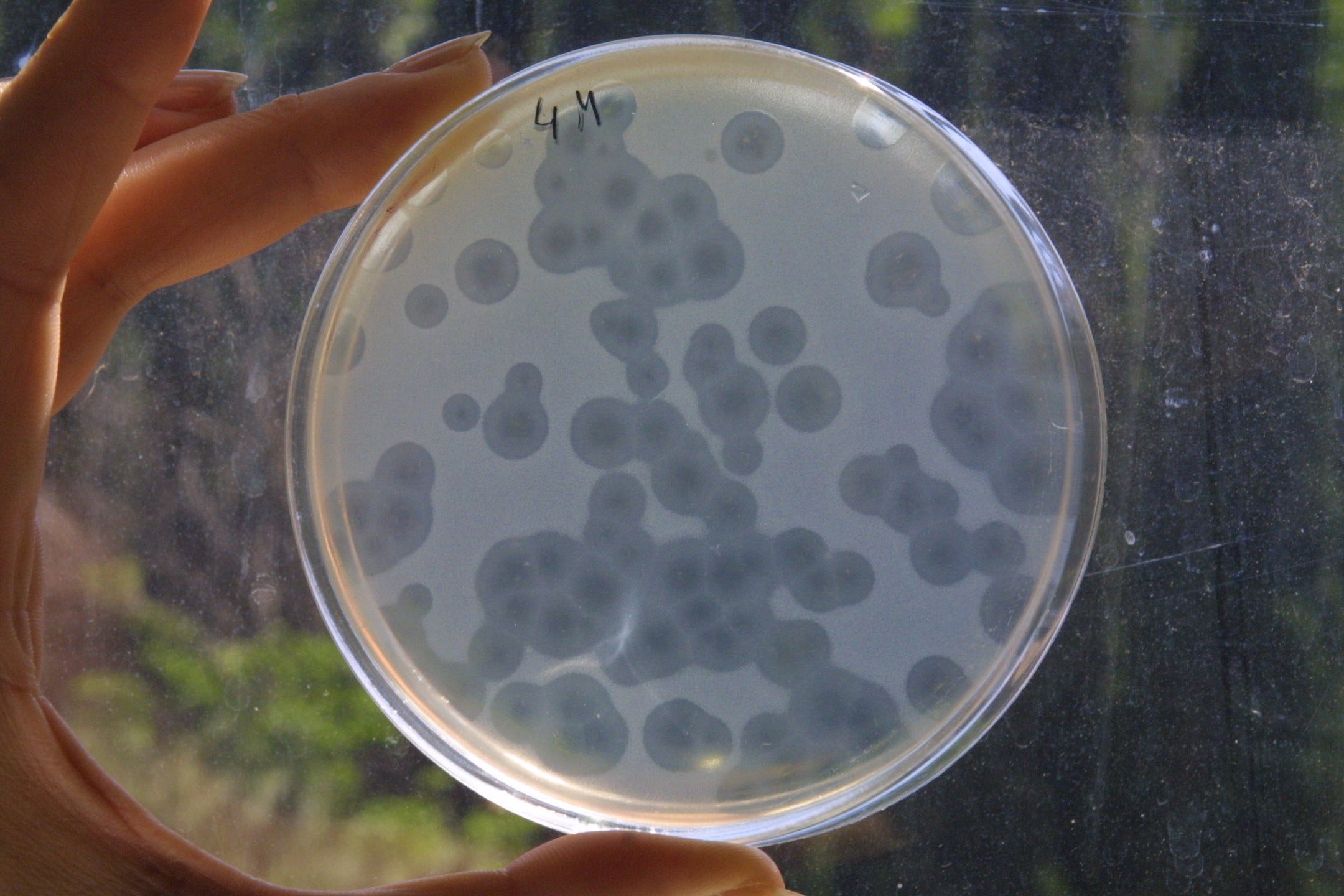
You simply sterilise your environmental sample to remove unwanted microorganisms (usually by filtration), which leaves just the phages behind. You then concentrate the sample by centrifuging it and plate it on to the bacterial host strain that you want to attack. You then look for the formation of plaques, which are multiple, small, 2mm blank circles or bull’s eyes against the grey-white covering of bacterial lawn spread evenly over the agar gel.
As antibiotic resistance issues worsen, scientific communities and biotech companies interested in the microbiome are starting to give more attention to phages. Bacteriophage therapy was intensely discussed at the most recent sixth Annual Microbiome Connect Europe Conference held remotely in Amsterdam in December last year.
Jonathan Solomon, CEO of BiomX, a biotech company in Israel presenting at the conference, is already doing phase two double-blind trials using phage cocktails. His team is developing these for a range of diseases such as acne, atopic dermatitis, inflammatory bowel disease and cystic fibrosis, diseases particularly affected by overuse of antibiotics causing dysfunction of the microbiome
He says: ‘It’s clear that antibiotic resistance is growing, but beyond a lot of talk I don’t see pharma and investors really stepping up.’
Reluctance to invest is not caused by the Food and Drug Authority (FDA) being awkward. In fact, it is positively encouraging phage research because phages are known to be extremely safe. “The FDA has publicly commented that with a phage product you don’t need to do animal safety or healthy volunteer studies. They’re very helpful and sort of forthcoming on phage therapy.”
Although phage is a natural product, he explains that a compound of phages can be patented. “It’s hard to get right. It’s not trivial. If the product is a cocktail, a compound, it’s a non-trivial composition. I don’t know if you can make money from it but it can definitely be patented!”
But Dr Soothill, a consultant microbiologist at Great Ormond Street Hospital, with experience on researching phage cocktails in patients, says: “Yes, you can get a patent, but the question is how effective is it going to be? Can someone alter it slightly and add in more phages and call it not your cocktail?”
There is a perfect window of opportunity right now for the government and private pharmaceutical companies to step in and sponsor an active phage library, laboratory and therapeutics centre in the UK
He has recently been involved in successfully treating a cystic fibrosis patient with a resistant Mycobacteria infection with individualised phage therapy. Approximately 10-12 patients have been treated in the US since 2016 using matched phages from the Pittsburgh Phage Library, but this was a patient at GOSH. Dr Soothill explains: “We did it, it was my idea to do it. The Pittsburgh people provided the phages.”
Although phage therapy is being used more, the difficulty is proving that it works well enough to convince the western scientific community to attract research grants and then to be able to generate more and better research. Properly assessing the case studies that have been done would help though. “If every time someone gave a phage to a patient, they measured phage multiplication and the number of bacteria around before and after, things would get on much faster and better. Not better than a big trial, but better than where we are.”
The lack of progress and the reluctance of western governments and companies to invest in the idea is frustrating. “I’ve been applying for infrastructure at GOSH. As things get worse in antibiotic resistance it may become easier to make the case for a phage therapy organisation …. And if it’s going to be lifesaving, they may pay quite a lot.”
Jonathan Solomon agrees. ‘It could be that you don’t need a phage department in every hospital, but you might have phage companies. Such as the group at Hebrew University of Jerusalem, which has been treating a dozen patients.
“You can see it bubbling in most countries because you have all of these antibiotic resistant cases that don’t respond and then some physician responds and organises compassionate care and everyone see the effect. It creates excitement.”
Some big pharma companies are at last investing in the technology. Edith Hessel, CSO of Eligo Bioscience, based in Paris, also presenting at the microbiome meeting says: “What’s so exciting is that with the bacteriophage, which naturally infect the bacteria, we can deliver a pay-load and literally put genes into the bacteria. And in that way, you can convert the bacteria into a more healthy state or you can use the bacteria to produce mediators that contribute to our health.” Eligo Bioscience must be persuasive. It has entered into a $224 million collaboration with GSK using these altered, and presumably patentable, phages with proprietary capabilities for treating acne.
There is a perfect window of opportunity right now for the government and private pharmaceutical companies to step in and sponsor an active phage library, laboratory and therapeutics centre in the UK. Something that has been grasped already by many other countries. Belgium has already made major progress with the Magistral Phage Medicine Strategy Authorisation rules, allowing individualised treatments to be formulated. The US has set up the Pittsburgh Lab, which now has over 15, 000 phages. And the Hebrew University of Jerusalem has developed a Clinical Phage Microbiology Framework to standardise laboratory protocols.
Antibiotic resistance is never going to go away – but nor will bacteriophages. If the UK acts quickly, we too could be at the forefront of this emerging bacteriophage technology and be one of the big players at the dawn of bacteriophage medicine.
Berenice Langdon is a GP and the author of ‘Learning Microbiology through Clinical Consultation’




Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments