The coming of the xenograft: A history of animal organ transplants
We’ve come a long way from the first human to human transplant in 1967, but the idea of using organs from animals is nothing new, explains Steven Cutts

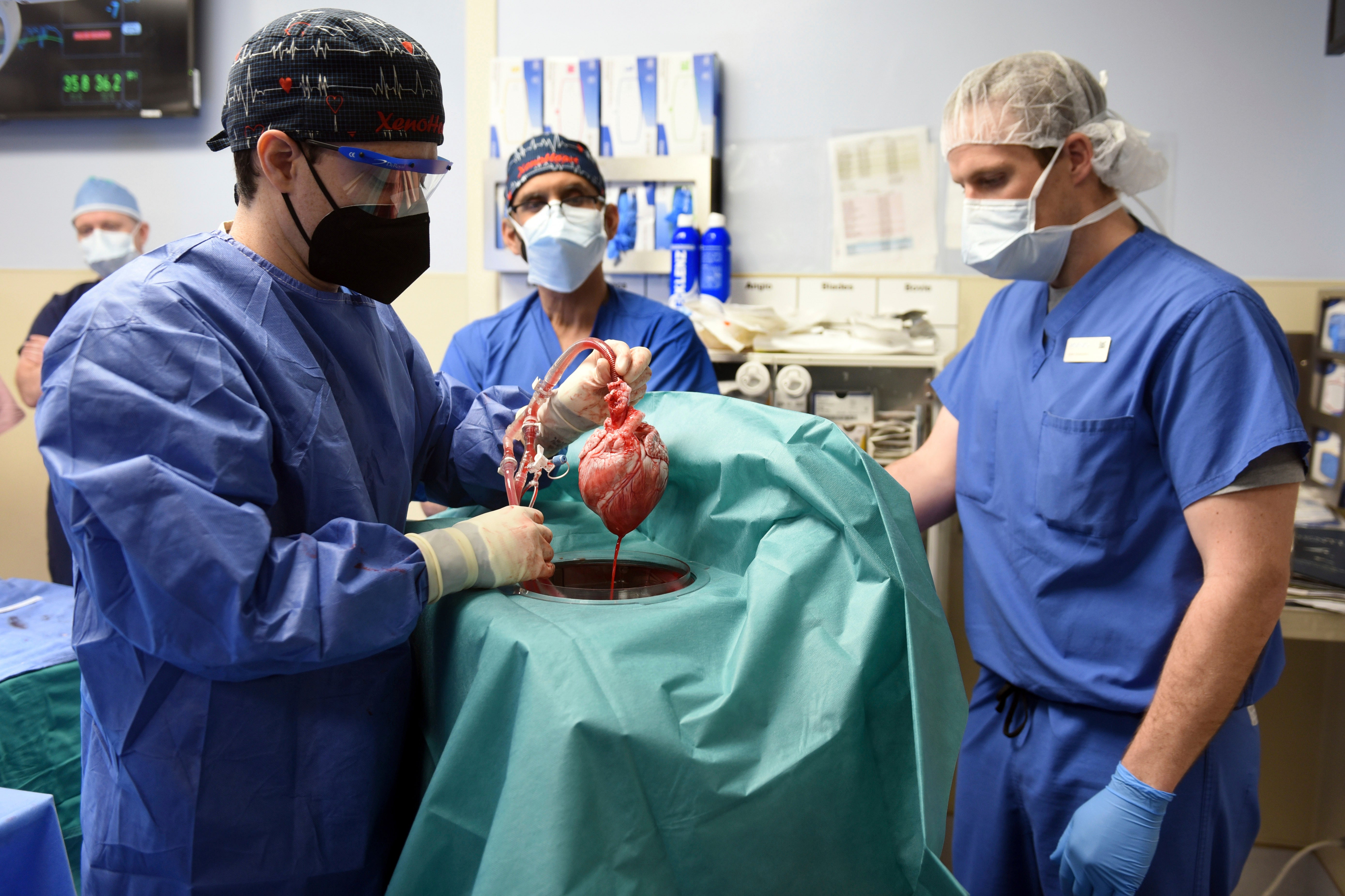
As I write these words, a man in America is alive and well with the heart of an animal still beating in his chest. What once was regarded as science fiction has suddenly become science fact.
Even in the best of circumstances, transplant surgery is not easy. The obstacles to success are too numerous to describe and as soon as one begins to contemplate organ transfer between two different species, the challenge becomes difficult to comprehend.
Organ transplants have been considered – and sometimes attempted – long before they had any real hope of working. In another age, the barriers to experimentation were much lower than they are today… back then, it didn’t seem to bother anyone too much when their experiments went wrong.
Early attempts at heart transplantation were mired in disaster. Plugging in another human being’s heart is relatively straightforward, but the attendant medical challenges are staggering. By the early 1970s, it was seen as acceptable to at least try. Given the huge economic cost of this kind of research, it still amazes me that they could find funding for a procedure that gained – at best – just a few months of life. This work was also delayed by widespread concerns about the very idea of transferring an organ like the heart from one person to another.
Since then, there have been government-backed campaigns to encourage organ donation. Unfortunately, as fast as we managed to de-stigmatise organ donation, we successfully stigmatised drunk driving, which was one of the main sources of donors. Parallel advances in car safety measures (seat belts, air bags, compulsory crash helmets, anti-lock brakes) made life increasingly difficult for a medic in search of an organ.
In the United States alone there are now 106,000 people waiting for organ transplants. About 6,000 of these will die every year. Of this number, the great majority are waiting for kidneys and many of the people with failing kidneys are beig treated in dialysis centres at great expense. The solution to this problem is simple: transplant animal organs into humans.
From a medical perspective, this is a formidable challenge.
Every cell in the human body carries its own ID card and our immune system is a perpetually vigilant border force, checking the ID on every cell in turn pretty much round the clock. In the human body, there are no undocumented workers at all. Any suggestion of a foreign agent in the midst and the immune cells go in, guns blazing. Needless to say, nothing will survive, certainly nothing resembling another person’s kidney, which clearly carries an ID card that bears no resemblance to the recipient’s own immune identity.
Early attempts at kidney transplantation focused on the issue of surgical technique, with one transplant after another failing before the surgeon had even closed the wound. Almost all of these disasters were almost due to rejection by the immune system. This failure to appreciate the significance of immune rejection is, perhaps, surprising given that doctors had managed to identify the main human blood groups, A, B, AB and O. Unless A-B-O compatibility is assured before a blood transfusion, the procedure is dangerous to the recipient. It seems to have taken a long time for people to recognise that a similar concept might apply to a solid organ.
By knocking out three specific genes, scientists had prevented the production of carbohydrates that would otherwise cause an antibody reaction in humans
Another solution would be to make use of man-made mechanical organs that can replace the function of the defunct organ. In the language of sci-fi, these would be bionic organs. Again, this is a lot harder than it sounds. There are machines that can substitute for the kidney for many years on end. There are also mechanical pumps that can replace the heart function, albeit for relatively brief periods of time. They need enormous support networks and consume huge amounts of fluid and consumables that we’d struggle to replicate inside the human body. As things stand, life on dialysis isn’t a lot of fun.
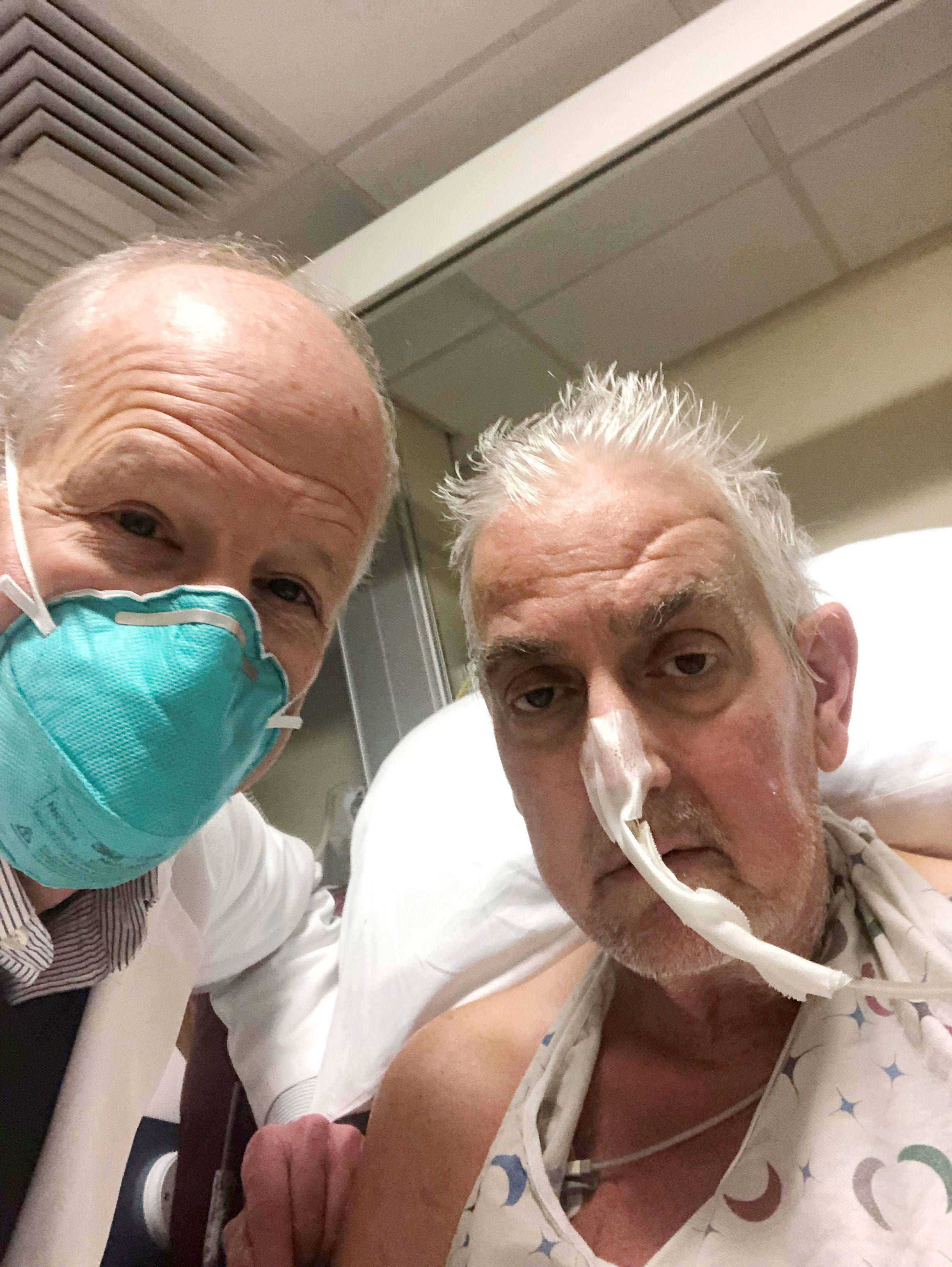
The scientific community has been anything but idle and there has been a continuous stream of research papers published in this field. Recently, there appeared to be a breakthrough in New York. A kidney from a genetically modified pig was sewn into a patient. The researchers found a person who was deemed to be brain dead, but still breathing on a life support machine. Next, they persuaded the relatives of the patient to give permission to implant an animal’s kidney into their body. The life support machine was finally turned off after 54 hours implantation. During this period, the organ had not been rejected. To be frank, two or three days of good function doesn’t prove anything very much at all, but it does represent evidence that an animal’s organ (with genetic modification) can survive in a human environment without immediate rejection.
Then in January this year, a successful animal to human heart transplant procedure was performed at the University School of Medicine in Baltimore. David Bennett is a 57-year-old patient and it remains to be seen how long he will survive.
The donor heart came from a pig that had been genetically modified. By knocking out three specific genes, scientists had prevented the production of carbohydrates that would otherwise cause an antibody reaction in humans. An additional gene was modified to prevent the heart from trying to grow inside David’s chest. Six human genes were then added to the pig’s genome to make it more receptive to the human environment.
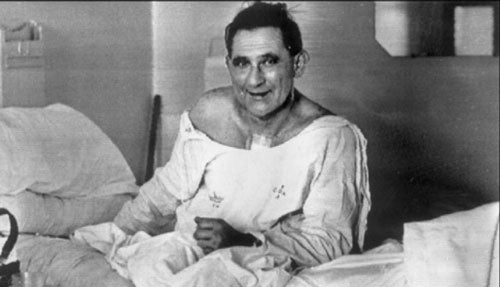
From a surgical perspective, this is hardly cutting edge. We should remember that the world’s first human to human heart transplant was performed as long ago as 1967 in Cape Town. Although many have forgotten the event entirely, it remains the single most publicised medical procedure in history. What isn’t so well publicised is the fact that the patient died from massive infection about 18 days later. Organ transplant required immunosuppressive drugs and that comes at a price. In practice, the entire field of organ transplantation would only became viable with the arrival of a new generation of drugs, in particular Cyclosporin, manufactured by the Swiss company Sandoz. Originally identified in a naturally occurring fungus, Cyclosporin was later synthesised and mass produced. It proved to be one of the biggest money earners for the company. While Cyclosporin came with side effects of its own, for patients who would have died without organ transplant, it was simply magical.
In short, overcoming immune rejection of the organ (whether it be human or animal) is the central challenge in transplant surgery.
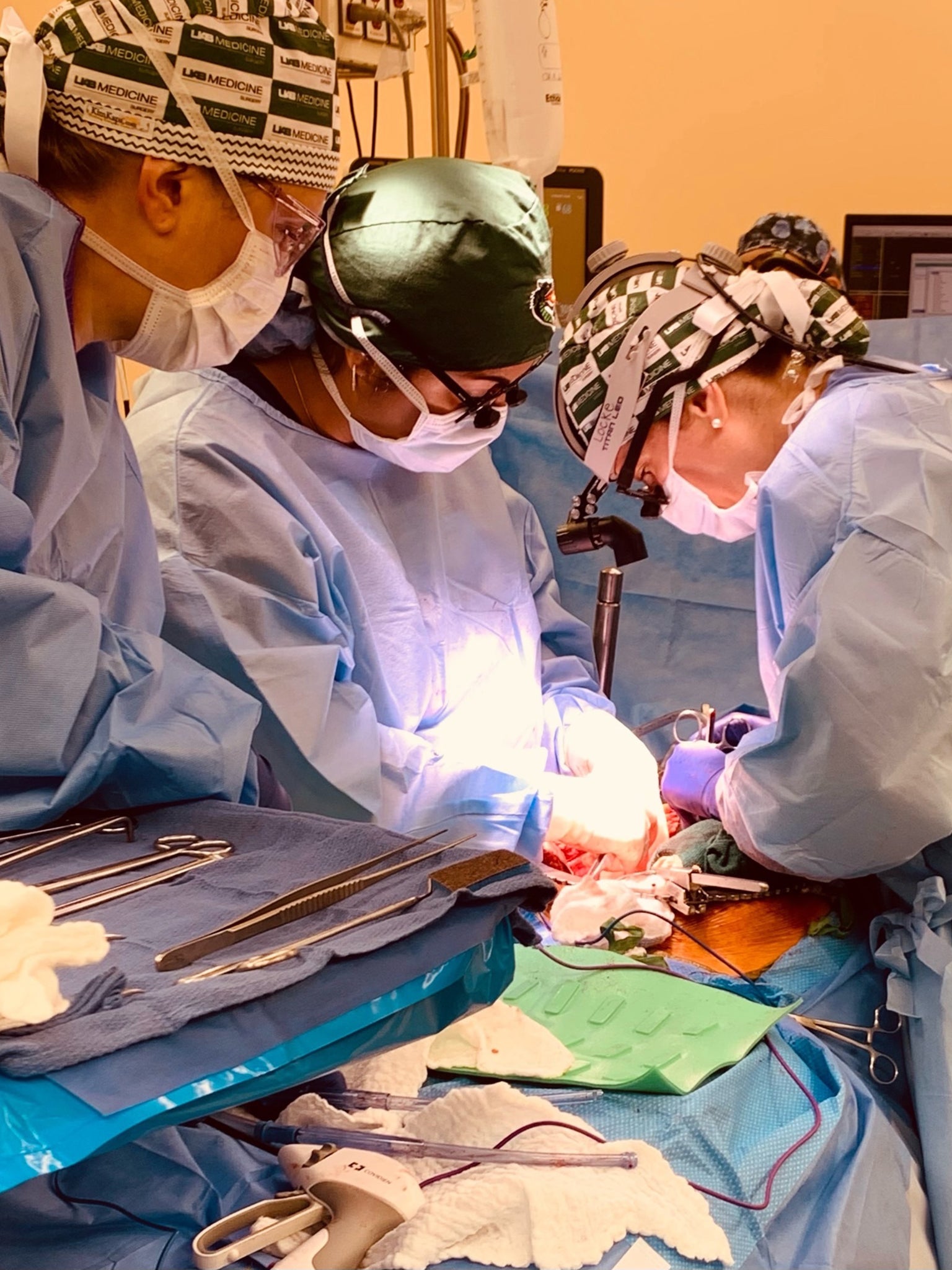
Given the obvious difficulty of finding a healthy human heart for transplant, scientists started to think about animal donors and in the first instance they looked relatively close relatives to man like chimps or baboons. However, it takes several years for a baboon to be large enough to provide a heart to an adult man. In contrast, a pig grows much faster than a human being or indeed any other primate. Pigs produce a large litter and each piglet is capable of reproduction before it is one year old. For these and other reasons, recent research has focused on the genetic modification of pigs.
What if we could treat people in kidney failure using animal organs alone? The people who manufacture dialysis machines would be pretty miffed but for society overall, it would be a real game changer
The American-based company Revivicor, a subsidiary of United Therapeutics, developed the genetically modified pig that was used for the recent procedure. It should be noted that there are now many biotech companies working in this field, spurred on by the promise of future transplant revenue on a massive scale. Doubtless they had staff prowling the corridors of every hospital in sight, looking for a patient who was suitable for a first trial. David Bennett has become a human guinea pig. He has simply gathered scientific information that could not be collected in any other way. If the data proves promising, many other men will become immensely wealthy long after this particular patient has died. It should be remembered that the American regulatory organisations only gave permission for the operation to be performed in the closing days of 2021.
The ethics of medical research have always been complex. Since this potentially groundbreaking operation was made public, it has emerged that many years ago Bennett assaulted someone with a knife, stabbing him repeatedly. Bennett went on to spend much of his own life in prison while his partially paralysed victim was confined to a wheelchair before dying of medical complications that were not amenable to transplant surgery. The researchers who performed his recent operation have made much of the fact that they have a duty of care to all patients, not judging them on any moral basis, but it’s hard to imagine he represents anything other than an opportunity to them.
There has long been concern that xenotransplantation (transplants between species) might be associated with the transmission of disease between two different species. It should also be noted that the ethical attitudes towards transplant vary between cultures. In Japan, for example, there are profound cultural objections to the transfer of organs between dead and living human beings. Given such constraints, surely animal to human transplantation would be doubly welcome in Japan?
Well, presumably they would be but we shouldn’t delude ourselves that the scientists involved are motivated purely by compassion. There is money to be made here too. Patients with renal failure are extremely expensive to manage. In 2008, it was estimated that the cost of treating people with renal failure in the UK amounted to 1-2 per cent of the total budget for the NHS. When we look at this figure, it’s important to remember that people with renal failure only represent 0.05 per cent of the general population.

But in the future, things may be different. What if we could treat people in kidney failure using animal organs alone? The people who manufacture dialysis machines would be pretty miffed, but for society overall, it would be a real game changer. Let’s face it, the amount of money we could save by closing down all the dialysis units in the world is staggering. Against this, remember that genetically modified animal organs won’t be cheap. The startup companies trying to develop these pigs have got where they are today by persuading very wealthy men to give them money for their research and not on a charitable basis.
In practice, there are other approaches to end-stage organ failure. For example, we could develop treatments that keep our own organs alive for longer. Other researchers looking at electromechanical organs that could be reliably implanted into a patient with intractable heart problems (aka “bionics” for Steve Austin fans). Some look at using human stem cells to populate a collagen scaffold and effectively manufacture a human heart identical to your own in genes and immune identity. In effect, the working heart you received would so closely resemble your own tissue that you probably wouldn’t even need immunosuppression drugs. This kind of work sounds promising, but in practice it remains very much lab-based. It’s the sort of thing we might still be talking about in another 15 years with no sign of a viable product.
The developments in Maryland are promising, but it’s hard to know how far we are from routine use of animal to human transplants. There’s a race to replace human organs on an industrial scale and when the xenografters finally get there, one or two other groups may have already beaten them to it.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments



Bookmark popover
Removed from bookmarks