How we’re dismantling cancer’s defences one brick at a time
More than 50 years after war was declared on cancer, real progress is starting to be made against humanity’s most complex disease, writes Samuel Lovett

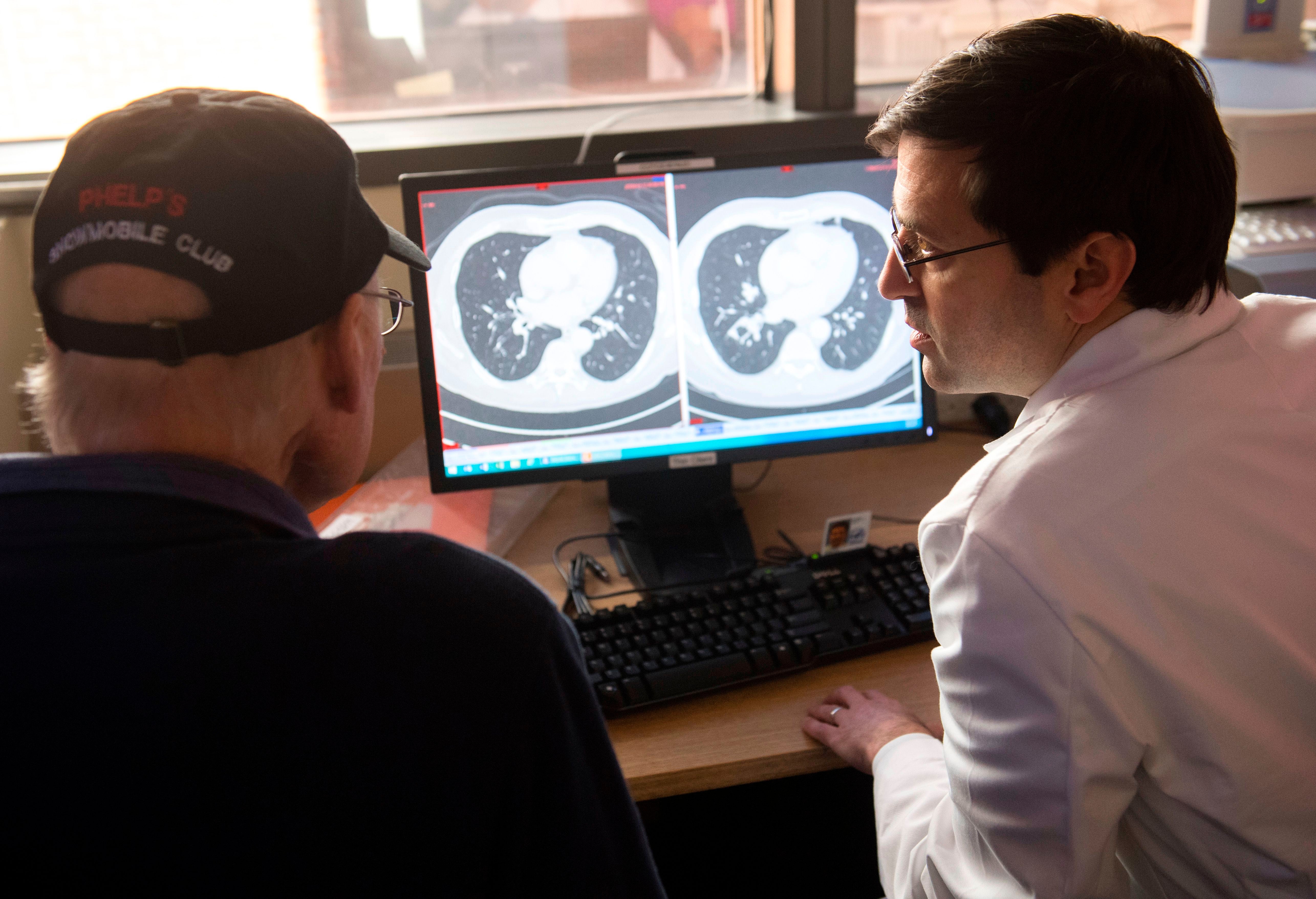
When it comes to treating cancer, the arc of progress has been long, slow and incremental. There have been plenty of breakthroughs along the way, but few have significantly shifted the dial. Speak to most oncologists, who deal in dollops of realism, not hyperbole, and they will always seek to temper expectations. There is no silver bullet – and nor will there ever be, they say.
But more than 50 years after the West first declared its “war against cancer”, the fruits of humanity’s labours are now falling from the trees in abundance.
Survival rates for the likes of breast, prostate and skin cancer are the highest they have ever been. Those with incurable forms of the disease are living longer. And, with each paper that is published, scientists better understand which hurdles need to be overcome to properly conquer cancer.
Of course, as the experts will hasten to add, there is a long way to go. But thanks to the ever-advancing progress of modern science, which is helping us fight cancer one step at a time, a clear path forward against the vastly complex disease is beginning to take shape.
In recent months, there has been talk of novel vaccines which train the immune system to destroy pancreatic cancer; immunotherapy which unmasks mutating cells in rectal cancer patients, making it easier for the body’s defences to destroy them; and drugs that slow the growth of cancerous breast cells once thought to be untreatable.
Most oncologists now believe that the right inroads are being made against cancer, raising hopes that, one day in the not-too-distant future, the disease will cease to be a death sentence for millions of patients.
“I don’t think we’re there yet,” says Dr Juanita Lopez, a clinical researcher at The Institute of Cancer Research. “But I think that is certainly the direction of travel.”
Dr Sam Godfrey, the senior research information manager at Cancer Research UK, is similarly optimistic. “We have an awful lot more technical proficiency, a lot of the technology has leapt forward, and it kind of feels like that we’ve finally got enough knowledge and technology to match the ambition to beat the disease.”
Much of the swelling excitement centres around the increasingly precise and tailored approach that scientists and medics can now take in treating the various forms of cancer.
“We’re starting to sort of salami slice the disease into different subgroups of patients who require perhaps different approaches, and are moving away from a one-size-fits-all approach,” says Dr Naureen Starling, a consultant medical oncologist at The Royal Marsden Hospital.
There is an understanding that no one cancer is the same. In every single patient, the disease emerges and is fuelled by a different set of mutations, which are hiding or evading from the immune system in their own unique way.
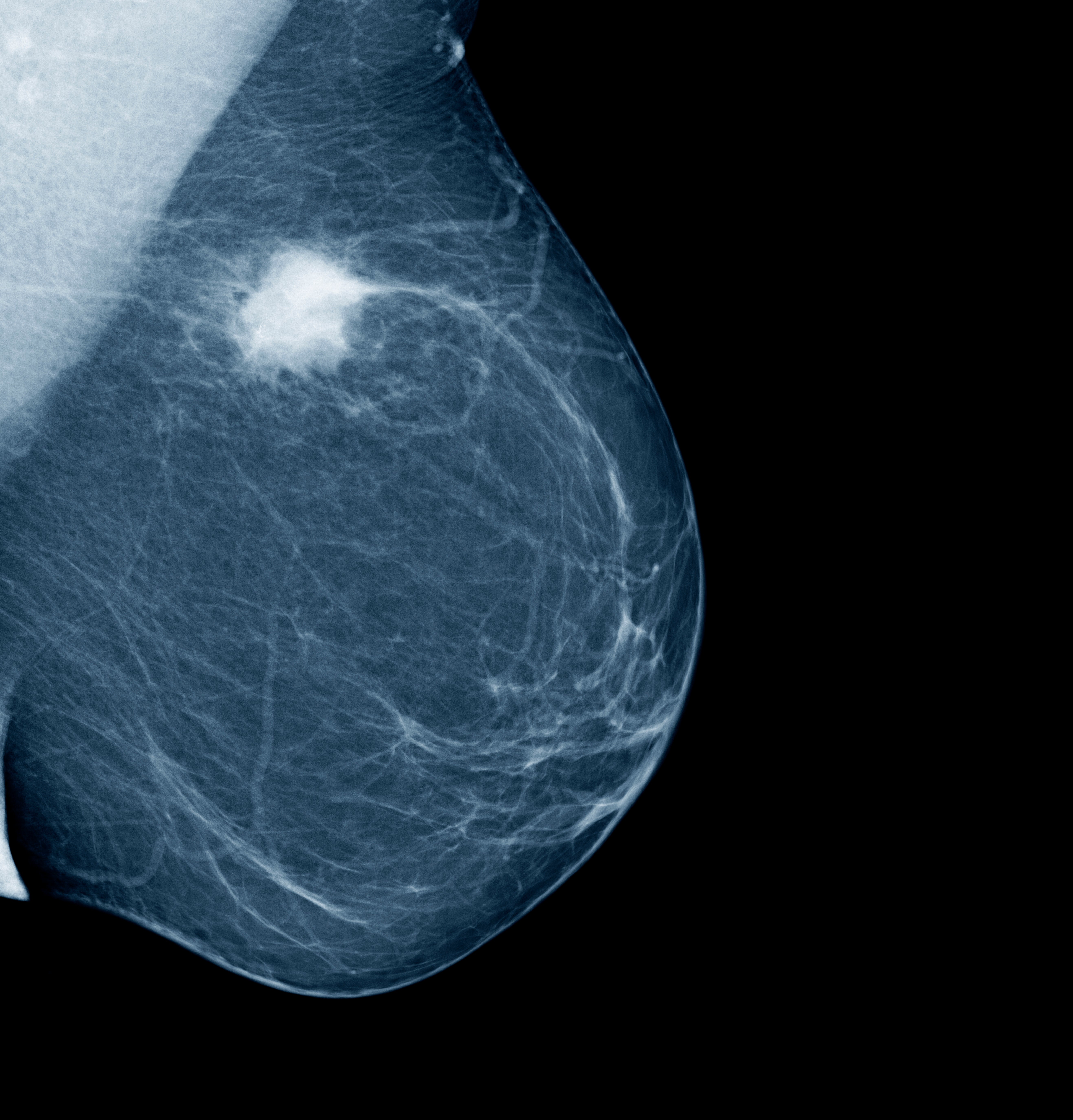
Oncologists will now look for certain genetic signals that could, for example, indicate whether they’re dealing with a rare and specific sub-category of cancer, one that accounts for a tiny proportion of patients but appears treatable.
Take the example of a recently-published study in which all 12 participants, who were suffering from rectal cancer, entered into remission after taking an experimental immunotherapy drug called dostarlimab.
This historic outcome – one of the scientists behind the research said, “this is the first time this has happened in the history of cancer” – meant the patients avoided the need for surgery, which can often be life-altering and disrupt bowel function.
Amid the excitement that surrounded this study, it was overlooked by many that all of the 12 patients in the study had tumours with a genetic mutation known as mismatch repair deficiency (MMRd), which is seen in a subset of approximately 5 to 10 per cent of patients with rectal cancer.
Patients with such tumours tend to be less responsive to chemotherapy and radiation treatments, which increases the need for surgical removal of their tumours.
Cancer has the ability to make itself invisible to our immune system, and there are all these different processes and circuitry that lead to this evasion
However, MMRd mutations can also make a tumour more vulnerable to the body’s immune system, especially when it’s bolstered by an immunotherapy agent – in this case, dostarlimab, which works by uncloaking previously invisible cancerous cells.
“Cancer has the ability to make itself invisible to our immune system, and there are all these different processes and circuitry that leads to this evasion,” says Dr Starling. “But immunotherapy tries to make that tumour more visible to the immune system, so it can be attacked and destroyed.”
This type of treatment has greatly improved outcomes for some of the worst cancers. Melanoma used to be untreatable; 20 years ago, patients would typically die within nine months of a stage-four diagnosis, says Dr Starling. But, thanks to the different immunotherapy drugs that have been developed over the years, 50 per cent of the same patients now survive.
Today, there are thousands of clinical studies underway seeking to harness the power of immunotherapy to exploit cancer’s soft spots. In the case of MMRd, the mutation is present in 4 per cent of advanced colon cancers, 4 per cent of stomach cancers, 2 per cent of pancreatic cancers and 4 per cent of small stomach cancers, says Dr Starling.

This raises the prospect that immunotherapy agents like dostarlimab could prove more widely effective, even if for a small proportion of patients. On a global level, if more sub-groups of patients are identified and treated with these emerging therapies, the number of saved lives will slowly accumulate.
“The [rectal cancer] study shows where these treatments are going,” says Professor Julian Downward, principal group leader and associate research director at the Francis Crick Institute. “It’s a bit early to say that that’s going to happen every time you treat a patient with this type of drug, but at least it’s quite a strong indication that these would have a really big impact.”
Of course, immunotherapy isn’t the answer for many patients, and understanding why some types of cancers do and don’t respond to this treatment remains a mystery. “There’s a Nobel Prize waiting for the person who has that answer,” says Dr Richard Simcock, a national clinical adviser at Macmillan.
Research suggests the effectiveness of a drug can depend on the location of a tumour and how the immune system interplays in that area. In some parts of the body, known as “immune deserts”, there is an absence of killer cells needed to combat the growth of cancer. The genetic variations underpinning a tumour also play a key role. Even the bacterial constitution of a patient is thought to have an influence.

“As an example, we now understand that cancer arising in the left side of the colon responds to certain therapies differently to cancer arising in the right side of the colon,” says Dr Simcock. “That’s one of the key challenges: figuring out which patients are going to benefit most from the most appropriate treatment.”
Targeted therapy is another tool that, thanks to modern science, is becoming increasingly refined and effective. This treatment focuses on the cancer itself, rather than the immune system, and will typically target cell switches and proteins involved in regulating division and replication that are misfiring, leading to uncontrolled growth.
A new study presented at the recent meeting of the American Society of Clinical Oncology (Asco) in Chicago, which was met with a standing ovation by those in the room, demonstrated the promise of one of these type of drugs, called trastuzumab deruxtecan, or Enhertu.
For patients who had metastatic breast cancer that had been progressing despite rounds of harsh chemotherapy, Enhertu targeted cancerous cells with laser-like precision, slowing tumour growth and extending life to an extent rarely seen with advanced cancers.
In the 373 patients who took trastuzumab deruxtecan, tumours stopped growing for about 10 months, as compared with five months for the 184 individuals who received standard chemotherapy.
The drug targets HER2, which is involved in normal cell growth and repair but can mutate to become over-expressed, helping to drive the spread of cancer. It’s a very common mutation in breast and other cancers, and for those with high levels of HER2, there are already a number of available drugs that can successfully treat it.
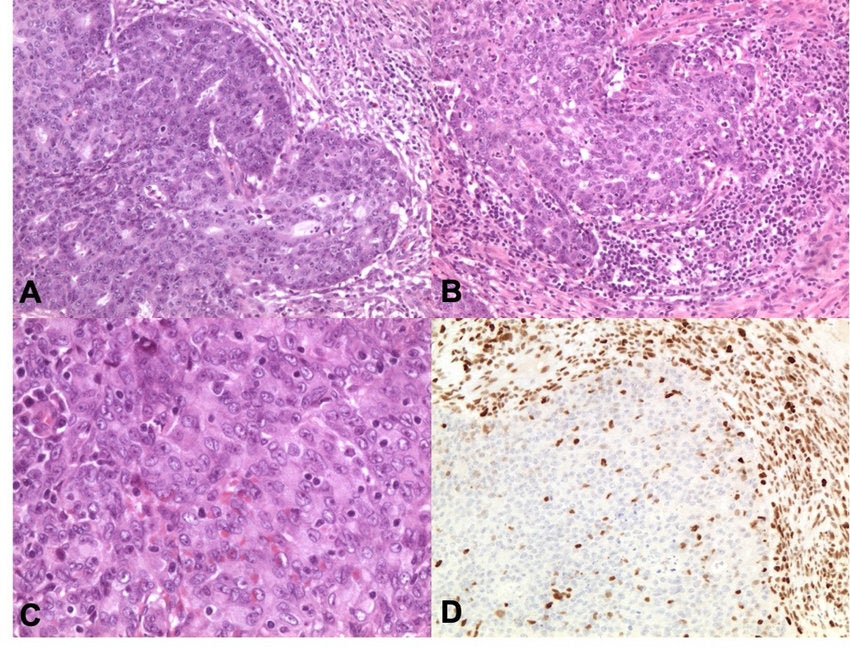
But for patients with only a few HER2 cells, such drugs are ineffective. Enhertu, however, appears to do the job. It contains an antibody that seeks out the HER2 protein and a chemotherapy drug that seeps into the cell itself, killing it, before moving into nearby cancer cells.
The implications of the research go beyond breast cancer and HER2. It now opens the door to targeting other molecular abnormalities on tumour cells that are only sparsely present in patients.
“This really is the business,” says Prof Downward. Other treatments, he adds, such as the emerging cancer vaccines, show promise but are still some way off from being accessible and deliverable.
He points to the example of a new vaccine which uses the same mRNA technology behind the Covid jabs to train the body’s immune system to kill pancreatic cancer cells.
In another study unveiled at Asco, half of the patients given the vaccine, designed to prevent cancer from returning after surgery, remained free of the disease 18 months later. Despite being one of the deadliest cancers, with 90 per cent of patients dying within two years of diagnosis, there has been no improvement in survival rates or treatment breakthroughs for decades.

Yet this latest research suggests there could be a way forward. The vaccine is tailor-made for each patient with mRNA genetic code found in their tumours. After being injected, the body is instructed to create proteins identical to those found on the surface of the tumours, triggering an immune response.
Any cancer cells displaying those same surface proteins that remain in the bloodstream after surgery are then destroyed by T cells, preventing the cancer from gaining a foothold. Out of 16 patients given the vaccine, administered in eight separate doses, half mounted an effective immune response and remained cancer-free throughout the study.
The trouble is, Dr Lopez explains, this “highly personalised” strategy is time intensive and complex, taking around four weeks to generate a vaccine for just one patient. It’s also “hugely expensive,” she says, which raises the question of whether such a treatment could feasibly be provided via the NHS.
“And the immune cells trained by the vaccine don’t always get into the cancer – and even if they do get in, they don’t actually eradicate the cancer,” Dr Lopez adds.
There’s much to take comfort and hope from. We have come a long way in the fight against the most complex disease that humans have ever faced
Prof Downward meanwhile estimates that it could take 10 to 20 years to determine whether the vaccine could be applied preventatively, like with the HPV jab, which has helped to dramatically cut cervical cancer rates in women.
Still, as with many other treatments, such an approach still opens the door to further advancements that, further down the line, could truly revolutionise efforts to treat and cure cancer.
But tackling the disease is much more about treating it or extending a patient’s life; it’s also about diagnosing it. And as any oncologist will tell you, the quicker a cancer is picked up, the higher the chances of survival.
With so much of today’s focus on genetics, research is underway to utilise this technology in detecting different types of the disease.
“Tumours as they grow and divide and change will release small amounts of DNA to the bloodstream,” says Dr Simcock. “We now have the ability to look for those pieces of DNA. We have the molecular equivalent of a giant magnet to find these very tiny needles in haystacks.”

The Galleri blood test, which is currently being trialled in the NHS, aims to detect these DNA traces and spot more than 50 kinds of cancers in people without symptoms. “The best way to cure a person with cancer is to get it early,” says Dr Simcock.
However, if the test proves successful, it will raise new questions and challenges for oncologists, he adds. “You’ve detected that you’ve got the cellular evidence of cancer, but when we do the things that we normally do to look for cancers and we don’t see anything, what does that mean for the patient? How should we follow them up? And how do we treat cancer this early on?”
In light of the developments we’ve seen over the last five decades, you would back the scientists to eventually answer such questions. Yet, there are other obstacles to overcome, too. Prof Downward is particularly concerned by the prospect of drug resistance in cancer and how this could undermine the advancements we’ve made against the disease.
“The problem we always come back to is that, especially for advanced cancers, they tend to be very genetically unstable and so generate a lot of mutations, some of which find ways around a therapy,” he says. “People have consistently underestimated this problem of cancer resistance.”

And while modern science has gotten better at treating localised cancers that aren’t overly advanced, our options against metastatic forms of the disease are limited. “When things spread all over the place, you just have too many cancer cells to be able to get rid of them all,” says Prof Downward. “The ones that are left tend to potentially develop resistance to the drugs.”
We remain similarly helpless in the face of the disease’s biggest killers, such as pancreatic, lung and brain cancer. Progress against these forms has been minimal, and some scientists fear the gap in survival rates between the deadliest and most treatable kinds of cancer will only widen in the years to come.
Nonetheless, there’s much to take comfort and hope from. We have come a long way in the fight against the most complex disease that humans have ever faced, and if the past 50 years are anything to go by, the future is likely to hold much more promise and potential.
While scientists don’t envision there will ever be a “eureka” moment when a blanket cure is discovered, there is a growing belief that cancer’s defences will be dismantled one brick at a time as treatments become ever more refined, ever more precise and ever more innovative. Slowly but surely, the scientists say, we will chip away at cancer until it no longer poses the threat it does today.
The successes we’re now seeing may be small, but they’re the “first step” to something much bigger, believes Dr Godfrey. “I think they’re probably analogous to the first steps we saw when chemotherapy started to come online. It could be the beginnings of a paradigm shift where we move to the next stage.”




Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments