The dirty truth about handwashing
The bacteria found on our hands is so individualised to us that it could be used instead for DNA to identify our presence at a crime scene. But that doesn’t mean we should be afraid of it, writes Berenice Langdon

I grip the key firmly to turn the lock to my clinic room, at the same time pressing down the door handle with my other hand. Using my forefinger, I press the light switch, switch on the computer and then use both hands on the keyboard for the control-alt-delete login.
I have just imprinted my unique hand microbiome all over the room and at the same time picked up some transient bacteria from my colleague with whom I share this office. This gives me a strange feeling of intimacy with her and her family. Less welcome to me is the collection of bacteria on the door handle which has necessarily been handled by every patient who has walked through the door. I am picking up a diverse collection of local bacteria each time I come into work, and then taking them home to my family.
But surely my clinic room is clean? And as a healthcare worker I must have clean hands? I must be washing my hands before every patient contact and again after. Presumably, I wash my hands with a disinfectant soap which means I have cleaned my hands of 99.9 per cent of germs?
Well yes. And no.
Yes, I wash my hands at the appropriate intervals as advised by the CDC and the World Health Organisation and all other medical bodies since 1975. And I wash them in the proper way, with liquid soap, patted dry with paper towels. But no, my hands will not, as a result of this, be free from bacteria.
More and more people are aware of the concept of “friendly bacteria” in our gut, and that these are “healthy”. It’s a step further to reveal that each and every square centimetre of our bodies is covered by bacteria; 10 million for every fingertip-sized area of skin.
What’s more, this is normal, and indeed necessary, for our survival in ways that are not fully understood. What is certain though, is that a healthy hand microbiome prevents pathogenic or harmful bacteria colonising. We are also coming to understand which bacteria are present in a healthy hand colony; over 150 species have been identified as typical.

As a woman, I am more likely to have Enterobacteriales, Moraxellaceae, Lactobacillaceae and Pseudomonaceae, while a man will have a subtly different selection. I will also have a more diverse population than is typical for a man. My dominant hand is more likely to have Peptostreptococcaceae and Xanthomonadales. In my case, this is my right hand, used in four of the five actions completed on entering my clinic room. As a healthcare worker, washing my hands sometimes 40 times a day, I am more likely to exhibit not just reduced diversity but, unfortunately, more pathogenic species than average, including pathogens such as S aureus, Enterococcus spp and Candida albicans.
Our hands are active communication devices, connecting our microbiome with those of other people, our families, our things and our houses
The impact of my work, my handling of patients and my handwashing has an impact on my personal microbiome which I then take home and imprint on my own house and my own family. Family members, even pets, all end up having many of the same bacterial taxa as each other.
And let’s say one of us committed a crime and by accident left our mobile phone at the scene. The individual nature of our hand microbiome left on the surface of our phones would be as powerful, if not more powerful, than analysis of our DNA to identify us.
Our hands are active communication devices, connecting our microbiome with those of other people, our families, our things and our houses. Our hands are also connecting our various body sites together and populating and repopulating each microbiome
Let’s go back to the clinic room. I have just turned on the tap and am squirting my hands with soap (again), having just examined a patient. Until now much handwashing research has focused on assessing and reducing bulk microbial load. The concept of hygiene has somehow become mixed up with the concept of sterilisation. But as we have seen, the idea of sterilising my hands after seeing my patient, and rendering them bacteria-free, is nonsense.

Hygiene relates to the “maintenance of health”. If activities to the hand such as hand-washing lead to the maintenance of good health, then this can be measured and proved by health outcomes, such as rates of upper respiratory tract infections, diarrhoeal illness or hospital-acquired (nosocomial) disease.
It is convenient for everyone who has engaged with the idea of a hand microbiome, myself included, to think in terms of bacterial “residents” and “transients”, the idea being that we wash the transients off between patients. Sometimes these terms are used interchangeably with “commensals” and “pathogens” as if all transients were pathogens, causing harm, and all residents were commensals.
However, the story is more interesting than this. Although some residents are commensals (microbes receiving benefit but no benefit to the human), others are mutualistic (both microbe and human receive benefit) and although some transients are pathogenic (causing harm to the host), others are not. Indeed it is possible to find some species of bacteria fulfilling all of these roles. For example, Staphylococcus epidermidis is often resident in the hand microbiome and usually regarded as a commensal. But it can be mutualistic, or occasionally act as a pathogen. Finally, it can be passed on to someone, say a newborn baby, in which case it would become a transient, before becoming a very necessary part of the baby’s resident new microbiome.
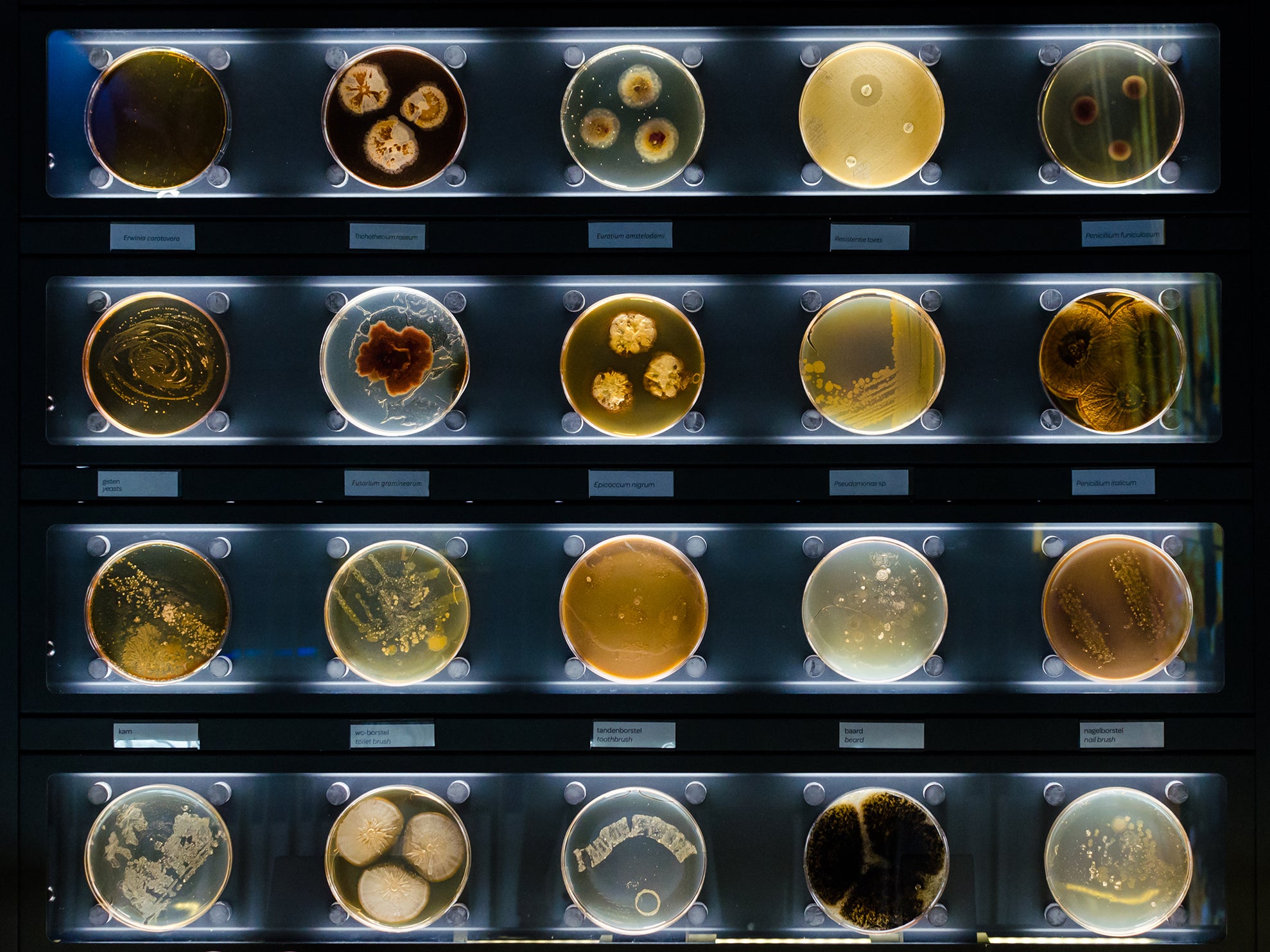
So far, we have only talked about superficial, hardy bacteria. But as new laboratory techniques develop, we are visualising the skin microbiome with greater depth and clarity. We can now take samples not only from the surface of the skin; the dermis and the epidermis, but deeper, right down to the fatty layers – which surprisingly have their own distinctive set of bacteria.
150
species of bacteria are typically found on our hands
Fast developing techniques of DNA sequencing with libraries of over 250,000 reference microbes mean that instead of just picking up the “weeds” of the bacterial world – the ones that grow on our petri dishes – the shyer bacteria can also now be picked out, those that will only grow with their friends or without oxygen.
Researchers are now talking as well about the “mycobiome” (fungi) and even the cutaneous “virome”. However, research is less straight forward here because of the smaller library of genetic material presently available. Viruses can also be particularly hard to pick out as their genetic material is so tiny; it can be swamped by the comparatively enormous bacteria and fungi.

In addition, we now have greater insight into how people’s bacteria change over time, with some researchers following up with patients for over two years. Others have compared microbiomes between humans living in different lifestyles – from city dwellers to hunter-gatherers – trying to work out what an authentic microbiome looks like. What was it like originally?
What has caused the loss of our skin microbiome species? Researchers speculate that a combination of factors may have been responsible. Daily bathing, increased caesarean rates, inappropriate use of disinfectants, chemicals in cosmetic products, even lack of contact with nature. All of these could play a part, as well as the obvious reason
Samples gathered from a group of semi-nomadic Native Americans showcase the microbiome of those living in a traditional society compared to US subjects. This group of people, living in a village in complete isolation from the west have a significantly higher skin microbiome diversity and not dominated by any one single species. In contrast, the group of US subjects analysed showed low skin microbiome diversity, dominated by the usual suspects; Staphylococcus, Corynebacterium, Neisseriaceae, and Propionibacterium (which causes acne). Some scientists estimate that we have lost over a third of our bacterial species.
What has caused the loss of our skin microbiome species? Researchers speculate that a combination of factors may have been responsible. Daily bathing, increased caesarean rates, inappropriate use of disinfectants, chemicals in cosmetic products, even lack of contact with nature. All of these could play a part, as well as the obvious reason; overuse of antibiotics.
Because no one really knows what the hand microbiome should look like it would seem difficult to develop a probiotic replacement. But that is exactly what some companies are trying to do. “Maybe its time to end the war on germs and rewild our skin!” suggests one brand of hand cream. Probiotics are formulations containing actual live bacteria to “seed” our skin with “beneficial probiotic species”. In contrast, hand creams with pre-biotics in them include products to feed the good bacteria, presumably at the expense of the bad.

To buy a hand cream which has both food for my hand bacteria and additional friendly bacteria to spread around the house (and presumably swallow sometimes) feels like a weird idea. What if I leave the cream on the window sill? Will the bacteria go mad and grow? Will they die? Although the idea of eating probiotic foods has become quite routine, the idea of probiotic hand products has not reached the same level of acceptability. But maybe one day it could be the saviour of both healthcare workers and patients alike.
In my clinic room I still have the tap running. I rinse off the soap and then dry my hands with six paper towels. Because my hands feel so uncomfortable, I apply an ordinary emollient and rub them together to moisturise. Messing up the hand microbiome is sometimes called dysbiosis and this is clearly what is happening to the skin on healthcare workers’ hands following their frequent handwashing. “The perturbation of microbial communities such that some bacteria shift to a pathogenic mode.” This is the official definition of dysbiosis and it certainly feels that way, as the hands of any healthcare worker will attest. No one really knows the impact of using different soaps or emollients on hygiene.
In fact, there are five “hygiene moments” listed for when healthcare providers should practice hand hygiene during patient care. These can really add up to a lot of visits to the sink. WHO advises not only washing hands before and after touching a patient but also after exposure to any “bodily fluids”, before a procedure such as an injection and finally, after touching the patient’s surroundings.
What about shaking hands when you meet your doctor? For most people this is a polite and courteous greeting. But for healthcare workers, a handshake can simply count as yet another patient contact but with an especially fertile area for microbes. For some it is a big no-no; embarrassing for both parties if the handshake is refused; if tolerated, leading to yet another handwash and further dysbiosis.
Handwashing is all we’ve got right now. It’s here to stay until we can think of something better. And we’ve got to do it better than the typical 40 per cent compliance rate found in healthcare settings. We must remember the seminal work of Semmelweis in 1861 linking enforced handwashing with a reduction in maternal deaths from puerperal fever; if they knew it then, then we must surely know it now. In the meantime, we all will just have to hang onto the notion that we are washing off the transient pathogenic bacteria and keeping rates of nosocomial disease to a minimum. Perhaps it is safer to simply forget the idea of a healthy hand microbiome for the present.
Berenice Langdon is a GP and author of ‘Learning Microbiology through Clinical Consultation’
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments