How ‘Made in India’ cough syrups ended up killing nearly 70 children in Gambia
Medicines had ‘unacceptable’ quantities of solvents found in several mass poisoning cases dating as far back as 1937

The deaths of 70 children in The Gambia from the consumption of four cough syrups manufactured in India have brought into the spotlight the medical manufacturing practices and exports of the country known as “pharmacy of the world”.
A preliminary investigation report by Gambian police on Tuesday said a total 69 children – most under five years of age – succumbed to acute kidney injuries which were linked to cough syrups made in India and imported via a US-based company.
The deaths in the west African country have been blamed on medicinal cocktails manufactured by Maiden pharmaceuticals situated in northern India’s Haryana state.
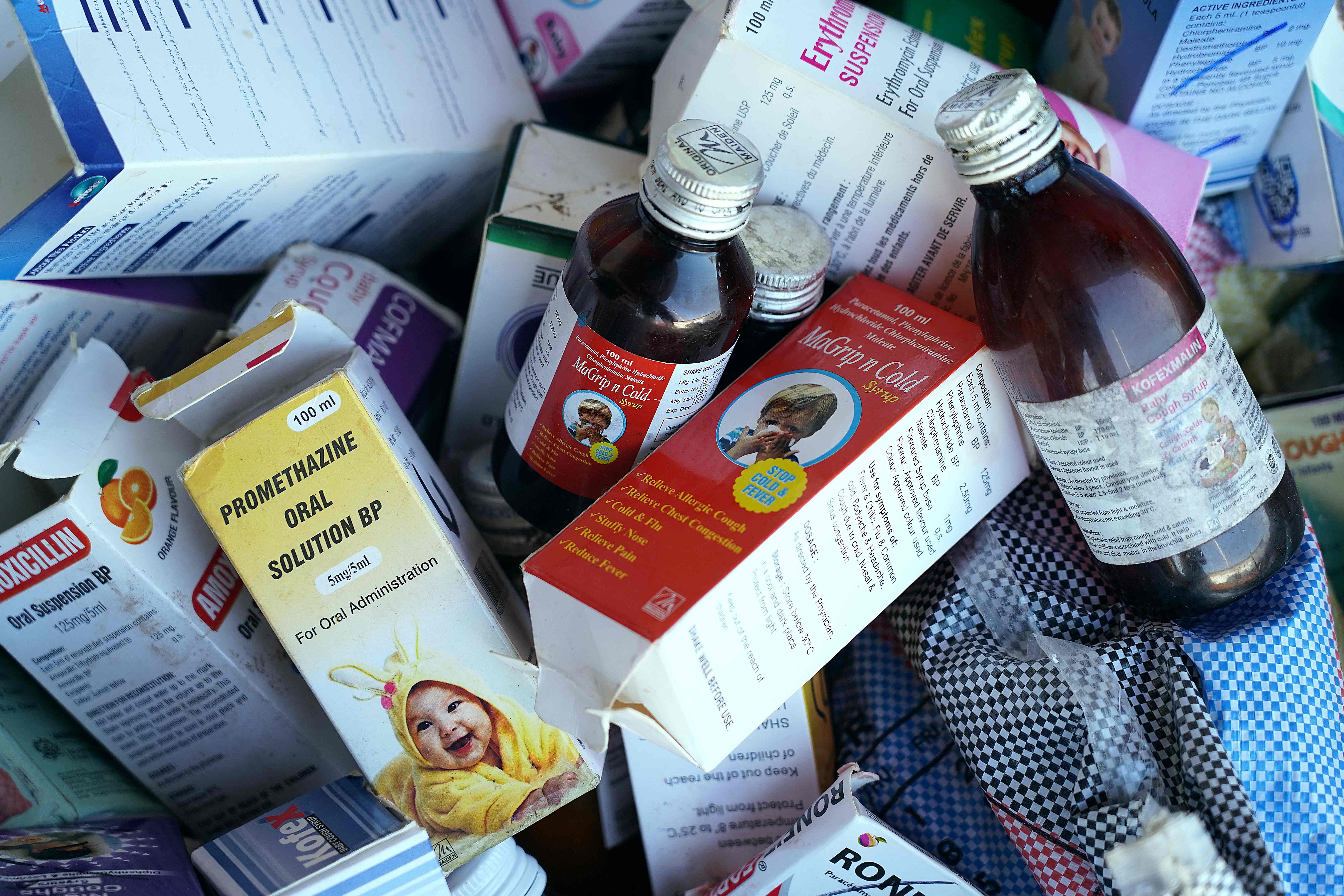
The cough syrups in question are Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup.
A lab analysis of these syrups showed “unacceptable” quantities of diethylene glycol and ethylene glycol, which can be toxic and cause acute kidney injury, the World Health Organization (WHO) said last week in a major setback to India, which is accountable for the production of a third of the world’s medicines.
All batches of the four products have been marked unsafe by the WHO until they can be analysed by the federal regulatory authorities, the UN’s health agency has ordered.
In response to the WHO report, Indian officials have halted the production of these cough syrups at one factory of Maiden pharmaceuticals after finding a dozen violations of good practices in an inspection.
Haryana state’s health minister Anil Vij said a factory near Sonipat town was inspected, following which they ordered the production of these syrups to be stopped.
The pharmaceutical company allegedly did not perform quality testing of propylene glycol, diethylene glycol and ethylene glycol.
Additionally some batches of propylene glycol did not have the manufacturing and expiry dates, reported Indian news website Moneycontrol.
Solvents diethylene glycol and ethylene glycol have their applications in antifreeze, brake fluids and other industrial uses. But they are also a cheaper alternative to glycerine in some pharmaceutical products, as the latter is used as a solvent or thickening agent in many cough syrups.
Identified as colourless and viscous liquids with a sweetish taste, the solvents have been found in several mass poisoning cases dating as far back as 1937.

According to Maiden, the cough syrup was approved for export only to The Gambia.
The company explained that it was not selling any of its products domestically in India. It added that officials at Maiden have been obtaining raw materials from certified and reputed companies.
Its website claims it was certified by the WHO and states it has an annual production capacity of 2.2 million syrup bottles, 600 million capsules, 18 million injections, 300,000 ointment tubes and 1.2 billion tablets at three factories.
But the Indian drug market is not alien to these toxic liquids causing fatalities either.
India’s pharmaceutical industry “has had a DEG [diethylene glycol] poisoning problem for a long time now, with the first event being reported in 1972 in Madras [present-day Chennai],” public health activist Dinesh Thakur, citing his upcoming book The Truth Pill: The Myth of Drug Regulation in India, told Indian daily Hindustan Times.
These poisoning events take place because many pharma companies in India do not test all excipients before they are used in manufacturing drugs, he said, adding that this happens despite a “Good Manufacturing Practice code expressly requiring such testing”.
Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies
Comments




Bookmark popover
Removed from bookmarks